Transcript
The consultants are paid speakers for Janssen Biotech, Inc. The speakers are presenting on behalf of Janssen and must present information in compliance with FDA requirements applicable to Janssen.
FRAME 1:
Dr. Hamilton:
Welcome to “The Man Cave” brought to you by Janssen Biotech, Inc. I am Dr. Hamilton. I'm a medical oncologist. I work at the Urology Centers Of Alabama. I’ll be your host for what is sure to be an informative discussion. I’m joined by Dr. Fabrizio and Dr. Taha.
Dr. Fabrizio:
Thanks for having me. My name is Dr. Michael Fabrizio, and I am a professor of Urology at Eastern Virginia Medical School and past CEO of Urology of Virginia.
Dr. Taha:
Thanks Dr. Hamilton. I’m happy to be here. I'm a medical oncologist at the Florida Cancer Specialists & Research Institute and Medical Oncology Director of Delray Medical Center.
Dr. Hamilton:
Today we’re going to discuss managing patients with metastatic castration-sensitive prostate cancer, or mCSPC, and highlight the TITAN study results for ERLEADA®, or apalutamide.
ERLEADA® is an androgen receptor inhibitor indicated for the treatment of patients with mCSPC and non-metastatic castration-resistant prostate cancer, or nmCRPC.
Before we dive in, it’s important to note that this program content is developed by Janssen Biotech, Inc. and the three of us are consultants who are paid speakers for Janssen Biotech, Inc. We are having this discussion on behalf of the company and are required to present information in compliance with the FDA requirements for communications about medicines, as applicable to Janssen Biotech, Inc.
Warnings and Precautions for ERLEADA® include cerebrovascular and ischemic cardiovascular events, fractures, falls, seizure, severe cutaneous adverse reactions, and embryo-fetal toxicity.
The most common adverse reactions greater than or equal to 10% that occurred more frequently in the ERLEADA®-treated patients greater than or equal to 2% over placebo from the randomized placebo-controlled clinical trials (TITAN and SPARTAN) were fatigue, arthralgia, rash, decreased appetite, fall, weight decreased, hypertension, hot flush, diarrhea, and fracture.
Dr. Hamilton:
Dr. Fabrizio and Dr. Taha, based on your extensive clinical experience treating men with prostate cancer at this stage, what do you consider as the critical goals in mCSPC management?
Dr. Fabrizio:
What I tell my patients is, our goal is to keep you alive longer while maintaining quality of life.
Dr. Taha:
For many physicians and patients, the most important treatment goal is to maximize overall survival, or OS. In addition, they may want to delay progression, castration-resistant disease, and chemotherapy.
Dr. Hamilton:
So, let’s take a look at their OS results with ERLEADA® in the TITAN trial.
For those who are unfamiliar, the TITAN trial was a phase 3, multicenter, randomized, double-blind, placebo-controlled trial of ERLEADA® plus androgen-deprivation therapy, or ADT, in patients with mCSPC. All patients received a concomitant gonadotropin-releasing hormone analog or had a prior bilateral orchiectomy.
FRAME 2 (Slide 1):
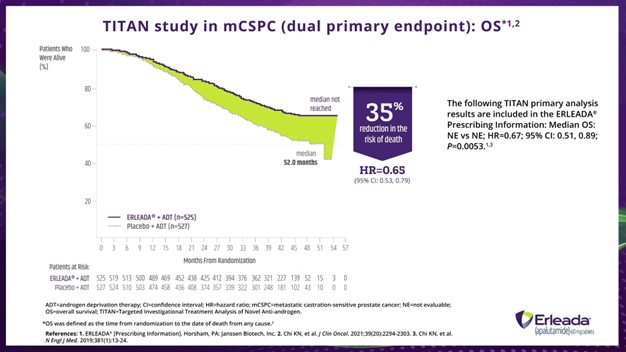
Dr. Hamilton:
In the final analysis of TITAN, after a median follow-up of approximately 4 years, ERLEADA® plus ADT significantly improved OS, with a reduction in the risk of death of 35% compared with ADT alone in a broad population of patients with mCSPC. So, a hazard ratio of 0.65, 95% confidence interval: 0.53 to 0.79. The median OS was not reached vs 52.2 months with placebo.
In the primary analysis, after a median follow-up of 22.7 months, treatment with ERLEADA® plus ADT reduced the risk of death by 33% compared with placebo plus ADT. So, a hazard ratio of 0.67, 95% confidence interval: 0.51 to 0.89, and a P value of 0.0053. Median OS was not estimable in either arm.
Footnotes:
ADT=androgen deprivation therapy; CI=confidence interval; HR=hazard ratio; mCSPC=metastatic castration-sensitive prostate cancer; NE=not evaluable; OS=overall survival; TITAN=Targeted Investigational Treatment Analysis of Novel Anti-androgen.
*OS was defined as the time from randomization to the date of death from any cause.
FRAME 3:
Dr. Hamilton:
This data helps to support the NCCN Clinical Practice Guidelines in Oncology® recommendation for apalutamide (ERLEADA®) plus ADT as one of the Category 1 preferred treatment options for patients with metastatic, or M1, castration-naïve prostate cancer; ADT alone is a category 2A other recommended intervention.
Dr. Taha:
So, Dr. Hamilton and Dr. Fabrizio, let’s say you’ve decided to put a patient on ERLEADA® plus ADT and you’re talking to them about your recommendation—how do you relay the overall survival data to them?
Dr. Fabrizio:
I usually bring in the data and explain that it indicates how this treatment works. I tell them that they may live longer with ERLEADA® plus ADT than if they were just on ADT alone.
Dr. Hamilton:
I tell them it’s not like your grandfather’s prostate cancer. Back when he was diagnosed, survival was more or less a year. But now, we have many treatment options that may achieve the goal of keeping you alive longer and delaying your progression or symptoms, while maintaining quality of life.
Dr. Taha:
What I always ask is, “If you knew that there was a medication that may increase your chance of survival with an established safety profile, wouldn’t you want to know about it?” And once they hear about it, most of my patients want to start treatment.
FRAME 4 (Slide 2):
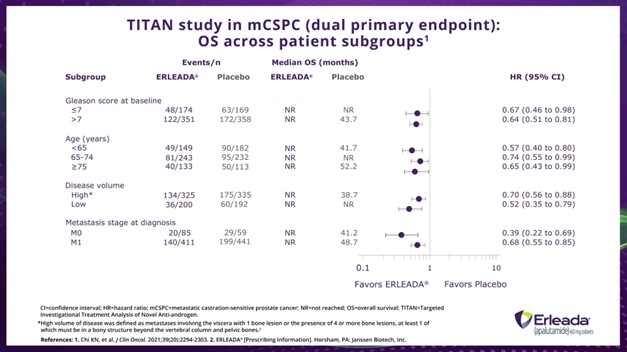
Dr. Taha:
When we look at OS across different subgroups, we see that ERLEADA® plus ADT demonstrated consistent improvement in OS versus placebo plus ADT regardless of Gleason score, age, disease volume, or metastasis stage at diagnosis.
Footnotes:
CI=confidence interval; HR=hazard ratio; mCSPC=metastatic castration-sensitive prostate cancer; NR=not reached; OS=overall survival; TITAN=Targeted Investigational Treatment Analysis of Novel Anti-androgen.
*High volume of disease was defined as metastases involving the viscera with 1 bone lesion or the presence of 4 or more bone lesions, at least 1 of which must be in a bony structure beyond the vertebral column and pelvic bones.
FRAME 5:
Dr. Fabrizio:
Previously there were limited options—now there are more options than just chemotherapy. So, it's all about educating physicians as well as patients that just because you have widespread disease it doesn't mean that you're not eligible for an advanced oral.
Dr. Hamilton:
Right, next let’s take a look at the radiographic progression-free survival, or rPFS, data.
FRAME 6 (Slide 3):
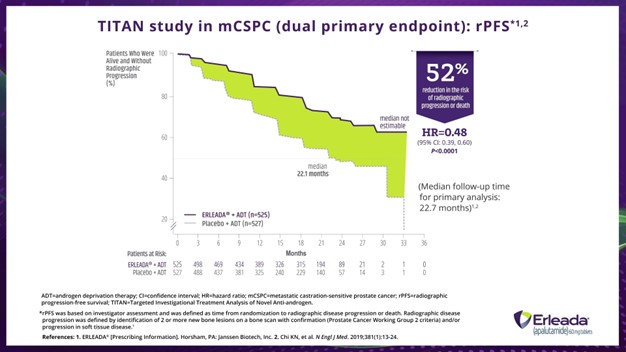
Dr. Fabrizio:
That’s a 52% reduction in the risk of radiographic progression or death with ERLEADA® plus ADT vs placebo plus ADT. Here we have a hazard ratio of 0.48, 95% confidence interval: 0.39 to 0.60, and a P value of less than 0.0001.
Dr. Taha:
Right. Here is a bit more information. At 2 years, 68% of patients treated with ERLEADA® plus ADT and 48% treated with placebo plus ADT were estimated to be alive without radiographic progression.
Footnotes:
ADT=androgen deprivation therapy; CI=confidence interval; HR=hazard ratio; mCSPC=metastatic castration-sensitive prostate cancer; rPFS=radiographic progression-free survival; TITAN=Targeted Investigational Treatment Analysis of Novel Anti-androgen.
*rPFS was based in investigator assessment and was defined as time from randomization to radiographic disease progression or death. Radiographic disease progression was defined by identification of 2 or more new bone lesions on a bone scan with confirmation (Prostate Cancer Working Group 2 criteria) and/or progression in soft tissue disease.
FRAME 7 (Slide 4):
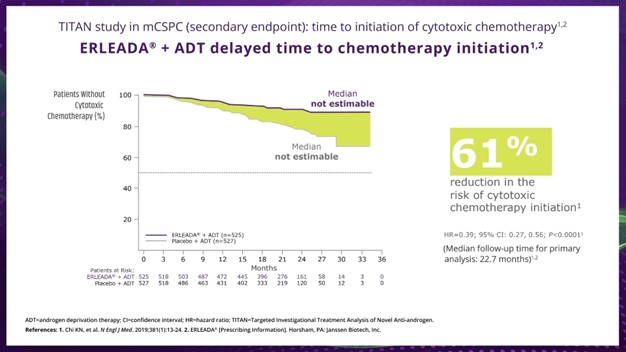
Dr. Taha:
So, now let's talk about the data time to cytotoxic chemotherapy. We see that ERLEADA® plus ADT significantly reduced the risk of chemotherapy initiation by 61% compared with placebo plus ADT in the primary analysis, with a median follow-up time of 22.7 months.
Footnotes:
ADT=androgen deprivation therapy; CI=confidence interval; HR=hazard ratio; TITAN=Targeted Investigational Treatment Analysis of Novel Anti-androgen.
FRAME 8:
Dr. Hamilton:
Dr. Taha, if you were to relay this information to your patients, how would you communicate it? And how do you think they’d react?
Dr. Taha:
I usually talk to my patients more about the disease continuum from localized to metastatic and emphasize that chemotherapy is usually reserved for more advanced disease, like metastatic castration-resistant prostate cancer. Starting ERLEADA® early in appropriate patients is the way to go. Dr. Fabrizio, don’t you agree?
Dr. Fabrizio:
Yeah. When I talk to my patients about all the treatment options available, some are reluctant about chemotherapy. I tell them that that’s normal. Telling them about the delay in the start of chemotherapy data is important.
Dr. Hamilton:
Absolutely. There are other excellent options that may extend survival. I let my patients know that chemotherapy can be offered at another time.
And last, but not least, we have time to castration resistance. Dr. Fabrizio, do you want to take this one?
FRAME 9 (Slide 5):
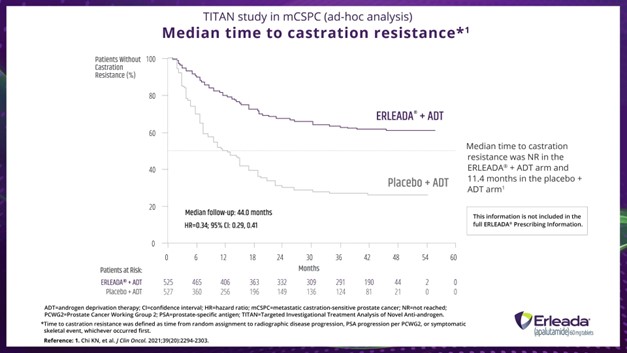
Dr. Fabrizio:
Of course. An ad hoc analysis of the TITAN trial showed that median time to castration resistance with ERLEADA® was not reached, but was 11.4 months for those on placebo plus ADT.
Footnotes:
ADT=androgen deprivation therapy; CI=confidence interval; HR=hazard ratio; mCSPC=metastatic castration-sensitive prostate cancer; NR=not reached; PCWG2=Prostate Cancer Working Group 2; PSA=prostate-specific antigen; TITAN=Targeted Investigational Treatment Analysis of Novel Anti-androgen.
*Time to castration resistance was defined as time from random assignment to radiographic disease progression, PSA progression per PCWG2, or symptomatic skeletal event, whichever occurred first.
FRAME 10:
Dr. Fabrizio:
I tell my patients that cancer cells eventually get used to not having testosterone. Eventually, and we don't know why, the tumor starts growing again. We call this hormone resistance or castration resistance.
FRAME 11 (Slide 6):
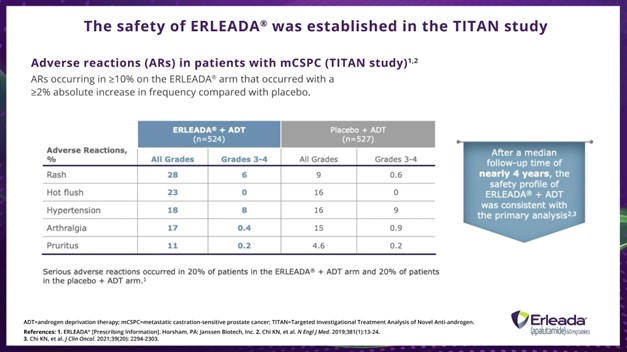
Dr. Hamilton:
The safety of ERLEADA® was established in the TITAN Trial. This slide shows adverse reactions (AR) occurring in greater than or equal to 10% on the ERLEADA® arm that occurred with a greater than or equal to 2% absolute increase in frequency compared with placebo in TITAN. In the TITAN study, serious ARs occurred in 20% of patients in the ERLEADA® plus ADT arm and 20% of patients in the placebo plus ADT arm. The safety profile of ERLEADA® plus ADT at 4 years remained consistent with that seen in the primary analysis.
Footnotes:
ADT=androgen deprivation therapy; mCSPC=metastatic castration-sensitive prostate cancer; TITAN=Targeted Investigational Treatment Analysis of Novel Anti-androgen.
FRAME 12:
Dr. Hamilton:
We talked through a lot of data today, but to bring us back to the top of our conversation—we said OS, delaying disease progression, delaying castration resistance, and delaying the start of chemotherapy were the critical goals in mCSPC management.
FRAME 13 (Slide 7):
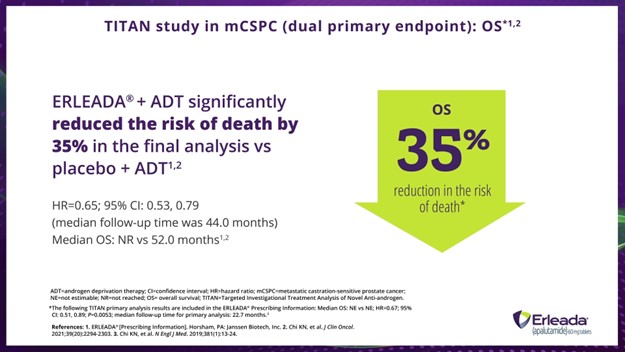
Dr. Hamilton:
In the TITAN study, compared with placebo plus ADT, ERLEADA® plus ADT reduced the risk of death by 35% versus placebo plus ADT in the final analysis.
Footnotes:
ADT=androgen deprivation therapy; CI=confidence interval; HR=hazard ratio; mCSPC=metastatic castration-sensitive prostate cancer; NE=not estimable; NR=not reached; OS=overall survival; TITAN=Targeted Investigational Treatment Analysis of Novel Anti-androgen.
*The following TITAN primary analysis results are included in the ERLEADA® Prescribing Information: Median OS: NE vs NE; HR=0.67; 95% CI: 0.51, 0.89; P=0.0053; median follow-up time for primary analysis: 22.7 months.
FRAME 14 (Slide 8):
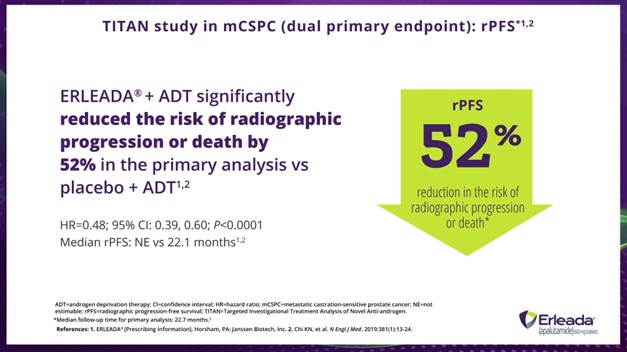
Dr. Hamilton:
In the primary analysis, ERLEADA® plus ADT reduced the risk of radiographic progression or death by 52%.
Footnotes:
ADT=androgen deprivation therapy; CI=confidence interval; HR=hazard ratio; mCSPC=metastatic castration-sensitive prostate cancer; NE=not estimable; rPFS=radiographic progression-free survival; TITAN=Targeted Investigational Treatment Analysis of Novel Anti-androgen.
*Median follow-up time for primary analysis: 22.7 months.
FRAME 15 (Slide 9):
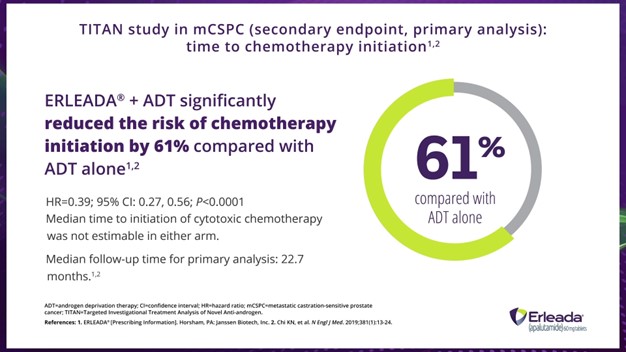
Dr. Hamilton:
Also, ERLEADA® plus ADT reduced the risk of chemotherapy initiation by 61%.
Footnotes:
ADT=androgen deprivation therapy; CI=confidence interval; HR=hazard ratio; mCSPC=metastatic castration-sensitive prostate cancer; TITAN=Targeted Investigational Treatment Analysis of Novel Anti-androgen.
FRAME 16 (Slide 10):
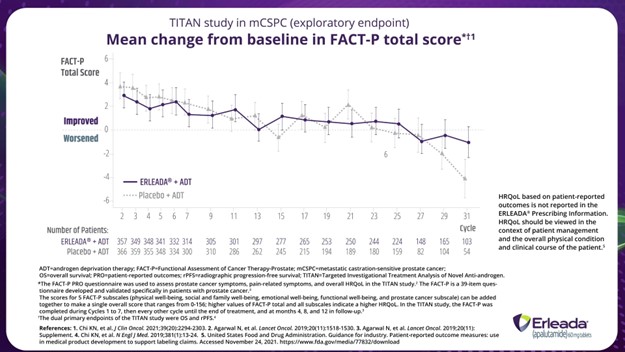
We won’t go through quality-of-life data in depth today, but quality of life was studied as an exploratory endpoint in the TITAN trial. Patients reported no negative impact to their health-related quality of life while taking ERLEADA® plus ADT. Median follow-up for time to pain-related endpoints ranged from 19.4 months to 22.1 months. There was also no difference in the experience of fatigue between treatment groups.
Footnotes:
ADT=androgen deprivation therapy; FACT-P=Functional Assessment of Cancer Therapy-Prostrate; mCSPC=metastatic castration-sensitive prostate cancer; OS=overall survival; PRO=patient-reported outcomes; rPFS=radiographic progression-free survival; TITAN=Targeted Investigational Treatment Analysis of Novel Anti-androgen.
*The FACT-P PRO questionnaire was used to assess prostate cancer symptoms, pain-related symptoms, and overall HRQoL in the TITAN study. The FACT-P is a 39-item questionnaire developed and validated specifically in patients with prostate cancer.
The scores for 5 FACT-P subscales (physical well-being, social and family well-being, emotional well-being, and prostate cancer subscale) can be added together to make a single overall score that ranged from 0-156; higher values of FACT-P total and all subscales indicate a higher HRQoL. In the TITAN study, the FACT-P was completed during Cycles 1 to 7, then every other cycle until the end of treatment, and at months 4, 8 and 12 in follow-up.
†The dual primary endpoints of the TITAN study were OS and rPFS.
FRAME 17:
Dr. Fabrizio:
We could have a whole other conversation about quality-of-life management in prostate cancer, but I’ll quickly say this—finding ways for patients to maintain their quality of life is a very important treatment goal.
Dr. Taha:
Right there with you, Dr. Fabrizio.
Dr. Hamilton:
Dr. Fabrizio and Dr. Taha, based on the data reviewed today, are both of you comfortable starting your patients on ERLEADA® early?
Dr. Taha:
Absolutely.
Dr. Fabrizio:
Yes.
Dr. Hamilton:
Great! A big thank you to Dr. Fabrizio and Dr. Taha for joining me today. Before we say goodbye, please stay with us as we go over the Important Safety Information for ERLEADA®.
INDICATIONS
ERLEADA® (apalutamide) is an androgen receptor inhibitor indicated for the treatment of patients with:
- Metastatic castration-sensitive prostate cancer (mCSPC)
- Non-metastatic castration-resistant prostate cancer (nmCRPC)
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Cerebrovascular and Ischemic Cardiovascular Events — In a randomized study (SPARTAN) of patients with nmCRPC, ischemic cardiovascular events occurred in 3.7% of patients treated with ERLEADA® and 2% of patients treated with placebo. In a randomized study (TITAN) in patients with mCSPC, ischemic cardiovascular events occurred in 4.4% of patients treated with ERLEADA® and 1.5% of patients treated with placebo. Across the SPARTAN and TITAN studies, 4 patients (0.3%) treated with ERLEADA® and 2 patients (0.2%) treated with placebo died from an ischemic cardiovascular event. Patients with history of unstable angina, myocardial infarction, congestive heart failure, stroke, or transient ischemic attack within 6 months of randomization were excluded from the SPARTAN and TITAN studies.
In the SPARTAN study, cerebrovascular events occurred in 2.5% of patients treated with ERLEADA® and 1% of patients treated with placebo. In the TITAN study, cerebrovascular events occurred in 1.9% of patients treated with ERLEADA® and 2.1% of patients treated with placebo. Across the SPARTAN and TITAN studies, 3 patients (0.2%) treated with ERLEADA®, and 2 patients (0.2%) treated with placebo died from a cerebrovascular event.
Cerebrovascular and ischemic cardiovascular events, including events leading to death, occurred in patients receiving ERLEADA®. Monitor for signs and symptoms of ischemic heart disease and cerebrovascular disorders. Optimize management of cardiovascular risk factors, such as hypertension, diabetes, or dyslipidemia. Consider discontinuation of ERLEADA® for Grade 3 and 4 events.
Fractures — In a randomized study (SPARTAN) of patients with nmCRPC, fractures occurred in 12% of patients treated with ERLEADA® and in 7% of patients treated with placebo. In a randomized study (TITAN) of patients with mCSPC, fractures occurred in 9% of patients treated with ERLEADA® and in 6% of patients treated with placebo. Evaluate patients for fracture risk. Monitor and manage patients at risk for fractures according to established treatment guidelines and consider use of bone-targeted agents.
Falls — In a randomized study (SPARTAN), falls occurred in 16% of patients treated with ERLEADA® compared with 9% of patients treated with placebo. Falls were not associated with loss of consciousness or seizure. Falls occurred in patients receiving ERLEADA® with increased frequency in the elderly. Evaluate patients for fall risk.
Seizure — In two randomized studies (SPARTAN and TITAN), 5 patients (0.4%) treated with ERLEADA® and 1 patient treated with placebo (0.1%) experienced a seizure. Permanently discontinue ERLEADA® in patients who develop a seizure during treatment. It is unknown whether anti-epileptic medications will prevent seizures with ERLEADA®. Advise patients of the risk of developing a seizure while receiving ERLEADA® and of engaging in any activity where sudden loss of consciousness could cause harm to themselves or others.
Severe Cutaneous Adverse Reactions — Fatal and life-threatening cases of severe cutaneous adverse reactions (SCARs), including Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) occurred in patients receiving ERLEADA®.
Monitor patients for the development of SCARs. Advise patients of the signs and symptoms of SCARs (eg, a prodrome of fever, flu-like symptoms, mucosal lesions, progressive skin rash, or lymphadenopathy). If a SCAR is suspected, interrupt ERLEADA® until the etiology of the reaction has been determined. Consultation with a dermatologist is recommended. If a SCAR is confirmed, or for other Grade 4 skin reactions, permanently discontinue ERLEADA®[see Dosage and Administration (2.2)].
Embryo-Fetal Toxicity — The safety and efficacy of ERLEADA® have not been established in females. Based on findings from animals and its mechanism of action, ERLEADA® can cause fetal harm and loss of pregnancy when administered to a pregnant female. Advise males with female partners of reproductive potential to use effective contraception during treatment and for 3 months after the last dose of ERLEADA®[see Use in Specific Populations (8.1, 8.3)].
ADVERSE REACTIONS
The most common adverse reactions (>10%) that occurred more frequently in the ERLEADA®-treated patients (>2% over placebo) from the randomized placebo-controlled clinical trials (TITAN and SPARTAN) were fatigue, arthralgia, rash, decreased appetite, fall, weight decreased, hypertension, hot flush, diarrhea, and fracture.
Laboratory Abnormalities — All Grades (Grade 3-4)
- Hematology — In the TITAN study: white blood cell decreased ERLEADA® 27% (0.4%), placebo 19% (0.6%). In the SPARTAN study: anemia ERLEADA® 70% (0.4%), placebo 64% (0.5%); leukopenia ERLEADA® 47% (0.3%), placebo 29% (0%); lymphopenia ERLEADA® 41% (1.8%), placebo 21% (1.6%)
- Chemistry — In the TITAN study: hypertriglyceridemia ERLEADA® 17% (2.5%), placebo 12% (2.3%). In the SPARTAN study: hypercholesterolemia ERLEADA® 76% (0.1%), placebo 46% (0%); hyperglycemia ERLEADA® 70% (2%), placebo 59% (1.0%); hypertriglyceridemia ERLEADA® 67% (1.6%), placebo 49% (0.8%); hyperkalemia ERLEADA® 32% (1.9%), placebo 22% (0.5%)
Rash — In 2 randomized studies (SPARTAN and TITAN), rash was most commonly described as macular or maculopapular. Adverse reactions of rash were 26% with ERLEADA® vs 8% with placebo. Grade 3 rashes (defined as covering >30% body surface area [BSA]) were reported with ERLEADA® treatment (6%) vs placebo (0.5%).
The onset of rash occurred at a median of 83 days. Rash resolved in 78% of patients within a median of 78 days from onset of rash. Rash was commonly managed with oral antihistamines, topical corticosteroids, and 19% of patients received systemic corticosteroids. Dose reduction or dose interruption occurred in 14% and 28% of patients, respectively. Of the patients who had dose interruption, 59% experienced recurrence of rash upon reintroduction of ERLEADA®.
Hypothyroidism — In 2 randomized studies (SPARTAN and TITAN), hypothyroidism was reported for 8% of patients treated with ERLEADA® and 1.5% of patients treated with placebo based on assessments of thyroid-stimulating hormone (TSH) every 4 months. Elevated TSH occurred in 25% of patients treated with ERLEADA® and 7% of patients treated with placebo. The median onset was at the first scheduled assessment. There were no Grade 3 or 4 adverse reactions. Thyroid replacement therapy, when clinically indicated, should be initiated or dose adjusted.
DRUG INTERACTIONS
Effect of Other Drugs on ERLEADA® — Co-administration of a strong CYP2C8 or CYP3A4 inhibitor is predicted to increase the steady-state exposure of the active moieties. No initial dose adjustment is necessary; however, reduce the ERLEADA® dose based on tolerability [see Dosage and Administration (2.2)].
Effect of ERLEADA® on Other Drugs
CYP3A4, CYP2C9, CYP2C19, and UGT Substrates — ERLEADA® is a strong inducer of CYP3A4 and CYP2C19, and a weak inducer of CYP2C9 in humans. Concomitant use of ERLEADA® with medications that are primarily metabolized by CYP3A4, CYP2C19, or CYP2C9 can result in lower exposure to these medications. Substitution for these medications is recommended when possible or evaluate for loss of activity if medication is continued. Concomitant administration of ERLEADA® with medications that are substrates of UDP-glucuronosyl transferase (UGT) can result in decreased exposure. Use caution if substrates of UGT must be co-administered with ERLEADA® and evaluate for loss of activity.
P-gp, BCRP, or OATP1B1 Substrates — Apalutamide is a weak inducer of P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and organic anion transporting polypeptide 1B1 (OATP1B1) clinically. Concomitant use of ERLEADA® with medications that are substrates of P-gp, BCRP, or OATP1B1 can result in lower exposure of these medications. Use caution if substrates of P-gp, BCRP, or OATP1B1 must be co-administered with ERLEADA® and evaluate for loss of activity if medication is continued.
Please see the full Prescribing Information for ERLEADA®.
References
- Agarwal N, McQuarrie K, Bjartell A, et al; TITAN Investigators. Health-related quality of life after apalutamide treatment in patients with metastatic castration-sensitive prostate cancer (TITAN): a randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2019;20(11):1518-1530. Accessed February 7, 2022. https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(19)30620-5/fulltext
- Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. 2021;39(20):2294-2303.
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med.2019;381(1):13-24.
- Data on File. Horsham, PA: Janssen Biotech, Inc.
- ERLEADA® (apalutamide) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Prostate Cancer V.3.2022. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed January 24, 2022. To view the most recent and complete version of the NCCN Guidelines®, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use, or application, and disclaims any responsibility for their application or use in any way.
- United States Food and Drug Administration. Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims. Accessed November 24, 2021. https://www.fda.gov/media/77832/download
© Janssen Biotech, Inc. 2023 08/23 cp-401240v1















