Prevalence of DED and Examining MGD
Walter O. Whitley, OD, MBA, FAAO
Pinning down the exact prevalence of dry eye disease (DED) has proven to be a challenge, in large part because the condition is one of the most underdiagnosed conditions in eye care.1 Approximately 16 million patients in the United States have been diagnosed with DED, but the total population of patients with DED could exceed 30 million to 34 million in the United States alone.2 Even though this population is high, only 1.5 million patients in the United States treat DED with a prescription medication2,3—which, using the lower end of the above US prevalence estimate of 30 million, means that only 5% of the total treatable population with DED in the United States uses a prescription for their condition.
Lack of awareness (from both patients and clinicians), misdiagnosis, asymptomatic presentation, and failure to discuss DED with patients all drive underdiagnosis. Real-world studies have highlighted the disconnect between prevalence, diagnosis and presentation of symptoms. For example, in the Prospective Health Assessment of Cataract Patients’ Ocular Surface (PHACO) study, 80% of patients presenting for cataract surgery had preexisting DED—but only 30% reported having occasional DED symptoms.4 Among those presenting in PHACO, only 22% of patients had been previously diagnosed with DED.
Misdiagnosis is a common issue in DED. Lemp reported that blurred vision or fluctuating vision—two symptoms associated with DED—were often misdiagnosed as refractive error or as symptoms secondary to cataract.5 The simultaneous presence of cataract and DED is possible; given that advanced age is a risk factor for DED, primary eye care providers often encounter patients with both DED and nascent or advanced cataract.2
In addition to age, a number of ocular and nonocular risk factors are linked with DED. Lid margin disease, history of contact lens wear, and history of ocular surgery are linked with higher rates of DED.2,3,6 Among the nonocular risk factors are female gender, use of systemic medication, use of topical medications that contain preservatives, and the presence of environmental irritants.2,3
Women, in particular, are at risk for DED, with a higher rate of severe symptomology and younger mean age of diagnosis.7 An uptick in rates of DED diagnosis among younger patients (ie, 18 to 34 years of age) of both sexes has been observed recently, perhaps due to increased exposure to digital screens and surgical face masks during spikes of airborne illness.2,8-11 Still, the difference in patients who are at least 50 years of age compared with those who are younger than 50 is stark: while only 3.4% of patients 18 to 49 have DED, approximately 11.3% of patients over 50 have DED.2
Clinical Examination of Signs and Symptoms
Not knowing where to begin an examination of a suspected DED patient is a challenge for primary eye care providers. Surveys and questionnaires such as DEQ-5, SANDE, SPEED, and OSDI may be useful in initial encounters with suspected DED patients, as they provide a baseline from which to assess symptoms.12-15 Personally, I rely on the SPEED questionnaire to facilitate conversations with newly presenting suspected DED patients. However, you don’t have to use a questionnaire. Asking common questions about DED symptoms can help identify those patients and can easily be performed by your technician team.
Other clinicians may prefer to initiate an examination with a conversation. The Tear Film and Ocular Surface Society (TFOS) Dry Eye Workshop (DEWS) II offers a list of potential triage questions for suspected DED patients. They include questions such as “Is your vision affected and does it clear on blinking?” and “Do you wear contact lenses?”16
Misalignment of DED signs and symptoms complicates diagnosis in clinical settings, presenting a challenge to real-world detection of disease.17 Some patients present with a clear list of DED symptoms, including ocular irritation, inconsistent vision, tearing, itching, and contact lens intolerance; others will present with no symptoms at all.3 A study of 263 patients with DED diagnoses found that only 57% of participants demonstrated symptoms consistent with DED.18 Conversely, some patients may present with clear symptoms of DED, but examination reveals inconsistent signs of disease. Reports in the literature show that half of patients with DED have no corneal staining but do show signs on Schirmer test and tear osmolarity scoring.19 Deferring to symptoms irrespective of signs may seem like a useful approach when observing this disconnect in the clinic,20 but relying merely on symptomatology is insufficient for diagnosis. For patients, these dynamics lead to frustration: they often feel that they cannot find a clinician who can help resolve their discomfort, leading to patients moving from practice to practice to find better options.
Signs and symptoms that are (or are not) observed upon presentation or diagnosis may change with time, with symptoms generally growing more intense the longer a patient has disease. Leinert et al reported that, among 784 patients with DED, worsening of ocular surface symptoms occurred in 24% of patients, worsening of vision-related symptoms occurred in 29% of patients, and worsening of social impact occurred in 10% of patients over a mean 10.5 years of disease duration.21 (Social impact scoring was based on questions regarding work satisfaction, socialization abilities and satisfaction, overall mood, and quality of relationships.) An analysis of a subset (n = 261) of patients in the same study who had enough clinical records for review revealed that approximately 46% of patients reported symptoms of fluctuating or blurred vision to at least one provider during the course of their disease; approximately 76% and 91%, respectively, reported discomfort and use of artificial tears during that same period.21

Meibomian Gland Dysfunction
DED is subtyped into aqueous-deficient disease and evaporative disease, each of which has different underlying etiologies.3 Primary eye care providers must execute an efficient and accurate differential diagnosis to determine which etiology drives disease in that particular patient.
These subtypes are not mutually exclusive: 36% of patients have both aqueous-deficient and evaporative disease. Only 14% of patients have exclusively aqueous-deficient DED, and 50% have exclusively evaporative DED.22-27 This means at a majority of patients (approximately 86%) have disease that is driven at least somewhat by evaporative pathology. Given that most evaporative DED is driven by meibomian gland dysfunction (MGD),23 and given that MGD often presents alongside primary aqueous-deficient DED, primary eye care providers must understand MGD’s role in driving DED.
Upon presentation, patients with MGD may report foreign body sensation, dryness, itching, and photosensitivity.17 MGD has been defined in the literature as:
[A] chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion. It may result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation, and ocular surface disease.28
Conditions that create blocked meibomian glands lead to a cascading event that ultimately exacerbates MGD. This feedback loop has led to MGD and DED being described as a vicious cycle.29 Blocked meibomian glands create conditions under which flora and microbes proliferate. This proliferation leads to increased production of lipases and esterases, which in turn increase the melting temperature for meibum.29 With an increased melting temperature, meibum is more likely to remain in a meibomian gland orifice, hardening and exacerbating preexisting blockage. Meibomian gland dropout also leads to a lipid layer deficit that increases tear film evaporation and invites hyperosmolarity and inflammation. The resulting keratinization of meibomian gland orifices worsens the meibomian gland blockages that triggered the cycle in the first place.29
If eye care providers can interrupt the vicious cycle described above, then patients may begin to experience relief.
By the time patients with MGD visit a clinic, they often have obstructed meibomian glands. Patients presenting for reasons other than obvious MGD often have MGD. To wit, 63% of pre-cataract surgical patients have MGD,4 as do 80% of glaucoma patients taking long-term antiglaucoma medications,30 and 60% of contact lens wearers.31 Primary eye care providers performing thorough eye care examinations should be vigilant for undiagnosed MGD, as catching disease early could be key to undercutting disease momentum.

Testing and Diagnostics for MGD
The TFOS DEWS II Diagnostic Test Battery provides a stepwise fashion that the group recommends for examination of suspected DED and MGD patients. Examinations start with a series of patient questions, and some providers choose to use questionnaires to capture these data. If a patient’s responses indicate that DED or MGD are possible diagnoses, providers can further probe whether risk factors such as smoking, age, and gender are present. These beginning steps can be the same for all primary eye care clinics, as they do not require specific technology or capital investment.
Diagnostic tests, however, are a different story. Most clinics to do not have every DED and MGD testing modality. However, we all have the basic tools of lid margin evaluation, corneal/conjunctival staining, and noninvasive tear break-up time (TBUT.) Other diagnostic testing modalities will be discussed later in this section.
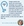
Meibomian gland examination should be performed after running diagnostic tests. Clinics with access to meibography should leverage such technology (Figure), and those that do not may wish to rely on a transilluminator to investigate a pulled-down eye lid. Relying on a severity scale to classify meibomian gland dropout may be useful for documenting progression of disease. Pult et al proposed a classification system that relies on estimated area of loss to stratify patients on a 0 to 4 severity scale (degree 1: <25% loss of meibomian glands, degree 2: 25% to 50%, degree 3: 51% to 75%, degree 4: >75%).32
If patients have obviously blocked meibomian glands, in-office expression of those glands may offer relief and will be part of the treatment discussion. Warming the lids prior to expression will ease the process and allow providers to examine the quality of meibum. Remember that although meibomian gland expression can reveal whether blocked meibomian glands are relevant to a patient’s disease, it cannot reveal much if the patient has significant gland dropout in the first place.
Characterization of meibum is an important part of meibomian gland assessment. While no single standard classification system exists to describe meibum quality, general determination of meibum’s viscosity and density can point us in the direction of a patient’s overall meibomian gland integrity. Providers who practice alone can determine their own scale for characterizing meibum, but those who practice in multiprovider clinics must establish a standardization scheme that can be used easily across providers.
 Figure. Meibography of a lid without meibomian gland atrophy shows consistent illumination and even distribution of glands (A). Early stages of meibomian gland dropout are observed in the center of the lid, which a patchy area of hypoillumination present (B). Prioritizing the meibomian gland health of a patient such as this one could be key to restoring ocular surface homeostasis. Significant meibomian gland dropout can be seen, with transillumination of nearly all tissue (C). Images courtesy of TearScience.
Figure. Meibography of a lid without meibomian gland atrophy shows consistent illumination and even distribution of glands (A). Early stages of meibomian gland dropout are observed in the center of the lid, which a patchy area of hypoillumination present (B). Prioritizing the meibomian gland health of a patient such as this one could be key to restoring ocular surface homeostasis. Significant meibomian gland dropout can be seen, with transillumination of nearly all tissue (C). Images courtesy of TearScience.Osmolarity and MMP-9 Testing
Many primary eye care providers who work with DED and MGD patients have access to osmolarity testing, which assesses the salt concentration of tears. This test must be placed carefully in the protocol of an examination, as it can only be performed at least 2 hours after any topically applied drops (including artificial tears) are used; the exception is topical anesthetic or dilating drops, which need only a 15-minute window from time of use to osmolarity testing. The test itself is rapid: within seconds, 50 nL of fluid are collected from the tear meniscus and results are displayed from the reader in under a minute. This intuitive modality can be used by technicians in many settings.
Rather than treating patients based on a single osmolarity score, it is advisable to track osmolarity over time. This provides a comprehensive sketch of a patient’s response to therapy or worsening of disease. Per the scale described by Starr et al, patients are considered normal if they have 280 mOsms/L to 300 mOsms/L on osmolarity scoring, have mild disease from 300 mOsms/L to 320 mOsms/L, moderate disease until they reach 340 mOsms/L, and have severe disease thereafter.33
Matrix metalloproteinase-9 (MMP-9) are proteolytic enzymes that are produced by stressed epithelial cells on the ocular surface.34 Although the presence of MMP-9s alone does not indicate the presence of disease (as MMP-9 detection is nonspecific to DED or MGD), use of MMP-9 detection technology is more sensitive than only using clinical signs, and the findings typically correlate with clinical exam findings.4 It may be difficult to acquire an adequate sample in patients with severe DED: the fabric used during sample collection must be wet enough to register with the device reader, and patients with severely dry eyes may require extra probing to secure a useful sample.
No single diagnostic test is sufficient to diagnose DED or MGD, just as no single symptom is sufficient. Part of the challenge facing primary eye care providers who diagnose DED and MGD is interpreting the constellation of datapoints acquired during examination, which sometimes conflict. Armed with as much data as possible, primary eye care providers can recommend therapeutic pathways after they diagnose and characterize disease.

1. Asbell PA, Spiegel S. Ophthalmologist perceptions regarding treatment of moderate-to-severe dry eye: results of a physician survey. Eye Contact Lens. 2010;36(1):33-38.
2. Farrand KF, Fridman M, Stillman IÖ, Schaumberg DA. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am J Ophthalmol. 2017;182:90-98.
3. Akpek EK, Amescua G, Farid M, et al. Dry Eye Syndrome Preferred Practice Pattern®. Ophthalmology. 2019;126(1):P286-P334.
4. Trattler WB, Majmudar PA, Donnenfeld ED, McDonald MB, Stonecipher KG, Goldberg DF. The Prospective Health Assessment of Cataract Patients’ Ocular Surface (PHACO) study: the effect of dry eye. Clin Ophthalmol. 2017;11:1423-1430. Published 2017 Aug 7.
5. Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995;21(4):221-232.
6. Sullivan DA, Rocha EM, Aragona P, et al. TFOS DEWS II Sex, Gender, and Hormones Report. Ocul Surf. 2017;15(3):284-333.
7. Matossian C, McDonald M, Donaldson KE, Nichols KK, MacIver S, Gupta PK. Dry Eye Disease: Consideration for Women’s Health. J Womens Health (Larchmt). 2019;28(4):502-514.
8. Moshirfar M, West WB Jr, Marx DP. Face Mask-Associated Ocular Irritation and Dryness. Ophthalmol Ther. 2020;9(3):397-400.
9. Jaiswal S, Asper L, Long J, Lee A, Harrison K, Golebiowski B. Ocular and visual discomfort associated with smartphones, tablets and computers: what we do and do not know. Clin Exp Optom. 2019;102(5):463-477.
10. Mehra D, Galor A. Digital Screen Use and Dry Eye: A Review. Asia Pac J Ophthalmol (Phila). 2020;9(6):491-497.
11. Giannaccare G, Vaccaro S, Mancini A, Scorcia V. Dry eye in the COVID-19 era: how the measures for controlling pandemic might harm ocular surface. Graefes Arch Clin Exp Ophthalmol. 2020;258(11):2567-2568.
12. Amparo F, Schaumberg DA, Dana R. Comparison of Two Questionnaires for Dry Eye Symptom Assessment: The Ocular Surface Disease Index and the Symptom Assessment in Dry Eye. Ophthalmology. 2015;122(7):1498-1503.
13. Ngo W, Situ P, Keir N, Korb D, Blackie C, Simpson T. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaire. Cornea. 2013;32(9):1204-1210.
14. Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye. 2010;33(2):55-60.
15. Begley CG, Caffery B, Chalmers RL, Mitchell GL; Dry Eye Investigation (DREI) Study Group. Use of the dry eye questionnaire to measure symptoms of ocular irritation in patients with aqueous tear deficient dry eye. Cornea. 2002;21(7):664-670.
16. Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15(3):539-574.
17. Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004;23(8):762-770.
18. Sullivan BD, Crews LA, Messmer EM, et al. Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: clinical implications. Acta Ophthalmol. 2014;92(2):161-166.
19. Sullivan BD, Crews LA, Sönmez B, et al. Clinical utility of objective tests for dry eye disease: variability over time and implications for clinical trials and disease management. Cornea. 2012;31(9):1000-1008.
20. Begley CG, Chalmers RL, Abetz L, et al. The relationship between habitual patient-reported symptoms and clinical signs among patients with dry eye of varying severity. Invest Ophthalmol Vis Sci. 2003;44(11):4753-4761.
21. Lienert JP, Tarko L, Uchino M, Christen WG, Schaumberg DA. Long-term Natural History of Dry Eye Disease from the Patient’s Perspective. Ophthalmology. 2016 Feb;123(2):425-433.
22. Badian RA, Utheim TP, Chen X, Utheim ØA, Ræder S, Ystenæs AE, Aakre BM, Sundling V. Meibomian gland dysfunction is highly prevalent among first-time visitors at a Norwegian dry eye specialist clinic. Sci Rep. 2021;11(1):23412.
23. Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472-478.
24. Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):71-81; quiz 82.
25. Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II Report Executive Summary. Ocul Surf. 2017;15(4):802-812.
26. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15(3):276-283.
27. Rabensteiner DF, Aminfar H, Boldin I, Schwantzer G, Horwath-Winter J. The prevalence of meibomian gland dysfunction, tear film and ocular surface parameters in an Austrian dry eye clinic population. Acta Ophthalmol. 2018;96(6):e707-e711.
28. Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922-1929.
29. Geerling G, Baudouin C, Aragona P, et al. Emerging strategies for the diagnosis and treatment of meibomian gland dysfunction: Proceedings of the OCEAN group meeting. Ocul Surf. 2017;15(2):179-192.
30. Uzunosmanoglu E, Mocan MC, Kocabeyoglu S, Karakaya J, Irkec M. Meibomian Gland Dysfunction in Patients Receiving Long-Term Glaucoma Medications. Cornea. 2016;35(8):1112-1116.
31. Machalińska A, Zakrzewska A, Adamek B, Safranow K, Wiszniewska B, Parafiniuk M, Machaliński B. Comparison of Morphological and Functional Meibomian Gland Characteristics Between Daily Contact Lens Wearers and Nonwearers. Cornea. 2015;34(9):1098-104.
32. Pult H, Nichols JJ. A review of meibography. Optom Vis Sci. 2012 May;89(5):E760-769.
33. Starr CE, Gupta PK, Farid M, et al; ASCRS Cornea Clinical Committee. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg. 2019;45(5):669-684.
34. Chotikavanich S, de Paiva CS, Li de Q, Chen JJ, Bian F, Farley WJ, Pflugfelder SC. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009;50(7):3203-3209.
In-Office Therapies for DED Related to MGD
Kelly K. Nichols, OD, MPH, PhD, FAAO
Part of the challenge to treating meibomian gland dysfunction (MGD) associated with dry eye disease (DED) is choosing the right intervention for each particular patient. We have numerous interventions at our disposal. In one sense, this means that we have multiple tools in our toolbox; in another sense, it means that we have so many choices that it can be difficult to keep them all straight.
In this section, I’ll explore which treatment options providers might consider for MGD-related DED, review some of the latest data related to those treatments, and preview a handful of datasets from investigational therapies.
Patient-Administered Treatments
For patients with lid margin disease, topical immunomodulators have been shown to provide a therapeutic benefit.1-5 Improvements in lipid layer parameters, tear production, visual acuity, corneal standing, and MGD have been observed following use of immunomodulators such as cyclosporine ophthalmic emulsion 0.05% (Restasis) and lifitegrast ophthalmic solution 5% (Xiidra).
Cyclosporine, in particular, has been used by primary eye care providers who treat DED for more than 2 decades. Regarding MGD, Kim et al have shown that ocular surface disease index (OSDI) scoring, tear breakup time (TBUT), and Schirmer test scoring improved at 3 months among patients who were dosed with cyclosporine compared with control.3
A formulation of water-free, pH-free cyclosporine 0.1% (Vevye) was approved for the signs and symptoms of DED in June 2023.6 In the pivotal Essense-1 and Essense-2 studies, patients with DED were randomly assigned to treatment or vehicle twice daily. The primary endpoint for both studies were the change in total corneal fluorescein staining (tCFS) at 4 weeks.4,7 Both studies met their primary endpoint. A responder analysis in Essense-2 suggested that the effect is clinically meaningful in 72% of patients in the treatment arm.7 The most common adverse event (8%) was instillation-site pain/pruritis.
In a 2020 study, Tauber et al compared lifitegrast therapy with thermal pulsation therapy in patients with MGD.4 The study authors found that at day 42, patients who were randomly assigned to lifitegrast therapy showed statistically significant improvements in eye dryness, corneal staining, and eyelid redness, and that a trend for improved BCVA from baseline was observed in the lifitegrast group. Given these findings, the authors recommended that providers include lifitegrast in a treatment regimen for patients with MGD.4
Topical steroids may be a good fit for some patients with MGD whose disease is driven by short bursts of inflammation. The safety and efficacy of loteprednol etabonate ophthalmic suspension 0.25% (Eysuvis) was evaluated in the phase 3 Stride study.8 In that study, treatment with loteprednol led to significantly improved ocular discomfort at day 15. Generic loteprednol is also sometimes used by providers off label.
Topical antibiotics are an option for DED and MGD. Topical azithromycin has both antibiotic and anti-inflammatory effects, and has been used off-label by providers for over a decade.9 Patients with lid margin disease in particular may benefit from topical azithromycin. Oral doxycycline has also been used for treating MGD and DED alongside physical expression of lids.10 Schlatter et al found that topical azithromycin was just as effective as oral doxycycline in patients with MGD, but had an overall better safety profile.11
Perfluorohexyloctane ophthalmic solution (Miebo), formerly known as NOV03, is a preservative-free semifluorinated alkane drop indicated for treatment of the signs and symptoms of DED. Rabbit eye models suggest that perfluorohexyloctane may penetrate meibomian glands at meaningful concentrations, leading to liquefaction of meibum at the orifice for the meibomian gland.12,13
The safety and efficacy of perfluorohexyloctane for the treatment of DED associated with MGD was assessed in the phase 3 Gobi and Mojave studies.14,15 Patients with DED and MGD were randomly assigned 1:1 to perfluorohexyloctane or saline (control) dosed four times daily, and the primary endpoints were the change from baseline in tCFS and eye dryness scoring at week 8. Both primary endpoints were achieved in both studies. Safety data were unremarkable, and can be closely examined in Figure 1.16,17 Using these data, the FDA approved the drug in May 2023.
Patients with Demodex mites may have Demodex blepharitis, which leads to MGD. Prior to approval of lotilaner ophthalmic solution 0.25% (Xdemvy), patients often turned to lid scrubs to manage Demodex infestation. However, we now have an FDA-approved option for these patients.
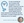
The efficacy and safety of lotilaner in patients with Demodex blepharitis were assessed the pivotal Saturn-1 and Saturn-2 studies.18-20 In those studies, patients were randomly assigned to treatment or vehicle and self-administered therapy twice daily for 6 weeks. A statistically significant reduction in complete collarette cure from baseline at day 43 (ie, the primary endpoint) was observed in both studies; the secondary endpoints of erythema cure and mite eradication composite cure based on complete collarette cure were also met in Saturn-1 and Saturn-2. Data findings for complete collarette cure and clinically meaningful collarette cure in Saturn-2 can be examined in Figure 2.
 Figure 1. The overall safety positive profile of perfluorohexyloctane in Gobi and Mojave found that blurred vision was the most common adverse event (AE). Rates of other AEs can be seen here. Note the saline (control) group sometimes saw higher rates for particular AEs. Adapted from Tauber J, et al. Presented at the 2022 American Society of Cataract and Refractive Surgeons Annual Meeting. Washington DC. April 22-26, 2022.
Figure 1. The overall safety positive profile of perfluorohexyloctane in Gobi and Mojave found that blurred vision was the most common adverse event (AE). Rates of other AEs can be seen here. Note the saline (control) group sometimes saw higher rates for particular AEs. Adapted from Tauber J, et al. Presented at the 2022 American Society of Cataract and Refractive Surgeons Annual Meeting. Washington DC. April 22-26, 2022. Figure 2. In Saturn-2, patients in the treatment arm experienced complete collarette cure in 56% of cases compared with 13% of cases in the vehicle arm at day 43 (P < .01); significant reductions were also seen on days 15 and 22. In addition, treatment patients experienced greater rates of clinically meaningful collarette cure at days 15, 22, and 43. Images courtesy of Tarsus Pharmaceuticals.
Figure 2. In Saturn-2, patients in the treatment arm experienced complete collarette cure in 56% of cases compared with 13% of cases in the vehicle arm at day 43 (P < .01); significant reductions were also seen on days 15 and 22. In addition, treatment patients experienced greater rates of clinically meaningful collarette cure at days 15, 22, and 43. Images courtesy of Tarsus Pharmaceuticals.In-Office Treatments
Thermal pulsation treatments have been shown to be effective at treating MGD. By heating and expressing meibomian glands in the office, patients can have the root cause of their disease addressed. The first FDA-approved, heat-based, in-office treatment for MGD, LipiFlow has been shown to effective up to 3 years after treatment in some patients.21 Providers are relatively hands off during a LipiFlow procedure, and rely on the physical device to warm lids and express glands.
The handheld iLux MGD Treatment System requires the provider to interface more directly with the patient. A light-based source of heat warms meibomian glands and, after glands are visualized through a magnifier, an expression tool in the device allows providers to release meibum. The second generation iLux device offers the same treatment approach, and adds meibomian gland imaging and video capabilities.
When compared in head-to-head studies, both devices have been shown to improve meibomian gland scores (MGS), TBUT, and OSDI scores.22-24 One study found that although iLux significantly improved MGS compared with LipiFlow, TBUT and OSDI scoring at week 1 and month 1 were not significantly different.24
Other in-office heat-based options include MiBo Thermoflo and TearCare. MiBo Thermoflo heats the lids and requires application of an ultrasound gel via a probe and does not contain an apparatus for gland expression; this step must be completed manually by the eye care provider who administers therapy. The device’s tips are not disposable and require cleaning.
TearCare allows localized heat to be applied to the patient’s lids during a 15-minute session, after which glands are manually expressed. The device’s stick-on applicators are disposable. In a comparison of TearCare and LipiFlow systems for patients with MGD, similar safety and efficacy were observed after a single session of either treatment.25 Another study, Sahara, compared TearCare sessions at baseline and month 5 to Restasis therapy self-administered twice daily, with follow-up at week 1 and months 1, 3, and 6. The primary endpoint was at month 6. It was determined that TearCare therapy led to significantly improved TBUT at all follow-up points.26 Significant differences favoring TearCare therapy were also observed at multiple follow-up points for meibomian gland secretion scoring, the number of glands yielding any liquid, and the number of glands yielding clear liquid (Figure 3).26 Given that real-world adherence to topical therapy is low,27 intervention with a heat-based platform such as TearCare may be appropriate in some patients.
Intraductal probing of meibomian glands to release obstructions in the gland orifice was described by Maskin in 2010.28 Although patient-reported rates of MGD relief following intraductal probing were as high as 100% in the literature, this procedure is invasive, requires specific training and instruments, and is uncomfortable for patients.
Intense pulsed light (IPL) treatment is a noninvasive procedure in which a nonlaser light source heats select vessels near the meibomian glands.29 In the literature, tear film stability and DED symptoms have resolved after IPL treatments with or without meibomian gland expression.30 OptiLight is the only approved IPL treatment for the management of MGD-related dry eye,31 and its use leads to improved TBUT, meibum quality, and gland expressability.32-34 Further, Suwal et al found that reduction of bacterial load and Demodex infestation occurs after IPL use.35
 Figure 3. In the Sahara study, localized heat therapy was significantly better than Restasis therapy at multiple timepoints when measuring differences in meibomian gland secretion score, the number of glands yielding any liquid, and the number of glands yielding clear liquid. Images courtesy Sight Sciences.
Figure 3. In the Sahara study, localized heat therapy was significantly better than Restasis therapy at multiple timepoints when measuring differences in meibomian gland secretion score, the number of glands yielding any liquid, and the number of glands yielding clear liquid. Images courtesy Sight Sciences.
The Pipeline
AZR-MD-001 is a novel formulation of selenium sulfide under investigation. AZR-MD-001 leverages 3 mechanisms of action—breaking down keratin disulfide bonds, slowing production of abnormal keratin, and stimulating meibum production—to address MGD. A phase 2b study met its coprimary endpoints, with significant increase in the number of meibomian glands yielding liquid secretions and a significant improvement in OSDI scoring.36 Treatment in the study was administered twice weekly at bedtime.36 A second pivotal study is underway, with results pending.36
Elevated levels of reactive aldehyde species (RASP) have been linked with DED.37 A topical formulation of a RASP inhibitor (Reproxalap) was assessed in the phase 3 Tranquility and Tranquility-2 studies.38,39 Although the primary endpoint was not met in Tranquility, a significant improvement in Schirmer test scoring was observed. Schirmer test scoring was then used as an endpoint in Tranquility-2, which met its primary endpoint. The company submitted regulatory filings with the FDA, and learned in November 2023 that the agency ruled that at least one additional study would be needed to demonstrate efficacy.40 The company is planning an additional phase 3 study in 2024.
1. Milner MS, Beckman KA, Luchs JI, et al. Dysfunctional tear syndrome: dry eye disease and associated tear film disorders - new strategies for diagnosis and treatment. Curr Opin Ophthalmol. 2017;27 Suppl 1(Suppl 1):3-47.
2. Rhee MK, Mah FS. Clinical utility of cyclosporine (CsA) ophthalmic emulsion 0.05% for symptomatic relief in people with chronic dry eye: a review of the literature. Clin Ophthalmol. 2017;11:1157-1166.
3. Kim HY, Lee JE, Oh HN, Song JW, Han SY, Lee JS. Clinical efficacy of combined topical 0.05% cyclosporine A and 0.1% sodium hyaluronate in the dry eyes with meibomian gland dysfunction. Int J Ophthalmol. 2018;11(4):593-600.
4. Tauber J. A 6-Week, Prospective, Randomized, Single-Masked Study of Lifitegrast Ophthalmic Solution 5% Versus Thermal Pulsation Procedure for Treatment of Inflammatory Meibomian Gland Dysfunction. Cornea. 2020;39(4):403-407.
5. Perry HD, Doshi-Carnevale S, Donnenfeld ED, Solomon R, Biser SA, Bloom AH. Efficacy of commercially available topical cyclosporine A 0.05% in the treatment of meibomian gland dysfunction. Cornea. 2006;25(2):171-175.
6. Novaliq Announces FDA Approval of VEVYE™ (Cyclosporine Ophthalmic Solution) 0.1%, for the Treatment of the Signs and Symptoms of Dry Eye Disease [press release]. Novaliq; June 8, 2023; Heidelberg, Germany and Cambridge, MA.
7. Akpek EK, Wirta DL, Downing JE, et al. Efficacy and Safety of a Water-Free Topical Cyclosporine, 0.1%, Solution for the Treatment of Moderate to Severe Dry Eye Disease: The ESSENCE-2 Randomized Clinical Trial. JAMA Ophthalmol. 2023;141(5):459-466.
8. Kala Pharmaceuticals Announces Statistically Significant Results for Primary and Key Secondary Endpoints in STRIDE 3 Clinical Trial Evaluating EYSUVIS™ for Signs and Symptoms of Dry Eye Disease [press release]. Kala Pharmaceuticals; March 9, 2020; Watertown, MA.
9. Foulks GN, Borchman D, Yappert M, Kakar S. Topical azithromycin and oral doxycycline therapy of meibomian gland dysfunction: a comparative clinical and spectroscopic pilot study. Cornea. 2013;32(1):44-53.
10. Paranjpe DR, Foulks GN. Therapy of meibomian gland disease. Ophthalmol Clin NA. 2003;16:37-42.
11. Schlatter A, Hommer N, Kallab M, et al. Effect of Treatment with Topical Azithromycin or Oral Doxycycline on Tear Film Thickness in Patients with Meibomian Gland Dysfunction: A Randomized Controlled Trial. J Ocul Pharmacol Ther. 2023;39(6):371-378.
12. ARVO 2018, Poster: Krösser et al; Ocular and systemic distribution of 14C-perfluorohexyloctane following topical ocular administration to rabbits.
13. Novaliq, data on file (F4H5).
14. Tauber J, Berdy GJ, Wirta DL, Krösser S, Vittitow JL; GOBI Study Group. NOV03 for Dry Eye Disease Associated with Meibomian Gland Dysfunction: Results of the Randomized Phase 3 GOBI Study. Ophthalmology. 2023;130(5):516-524.
15. Sheppard JD, Kurata F, Epitropoulos AT, Krösser S, Vittitow JL; MOJAVE Study Group. NOV03 for Signs and Symptoms of Dry Eye Disease Associated With Meibomian Gland Dysfunction: The Randomized Phase 3 MOJAVE Study. Am J Ophthalmol. 2023;252:265-274.
16. Tauber J, et al. Presented at the 2022 American Society of Cataract and Refractive Surgeons annual meeting, April 22-26, 2022.
17. Sheppard JD, Kurata FK, Epitropoulos A, et al. Efficacy of NOV03 (Perfluorohexyloctane) on Signs and Symptoms of Dry Eye Disease associated with Meibomian Gland Dysfunction: The Mojave study. Invest Ophthalmol Vis Sci. 2022;63:1531.
18. Yeu E, Wirta DL, Karpecki P, Baba SN, Holdbrook M; Saturn I Study Group. Lotilaner Ophthalmic Solution, 0.25%, for the Treatment of Demodex Blepharitis: Results of a Prospective, Randomized, Vehicle-Controlled, Double-Masked, Pivotal Trial (Saturn-1). Cornea. 2023;42(4):435-443.
19. Gaddie IB, Donnenfeld ED, Karpecki P, et al; Saturn-2 Study Group. Lotilaner Ophthalmic Solution 0.25% for Demodex Blepharitis: Randomized, Vehicle-Controlled, Multicenter, Phase 3 Trial (Saturn-2). Ophthalmology. 2023;130(10):1015-1023.
20. XDEMVY [prescribing information]. Tarsus Pharmaceuticals, Inc; 2023.
21. Greiner JV. Long-Term (3 Year) Effects of a Single Thermal Pulsation System Treatment on Meibomian Gland Function and Dry Eye Symptoms. Eye Contact Lens. 2016;42(2):99-107.
22. Tauber J, Owen J, Bloomenstein M, Hovanesian J, Bullimore MA. Comparison of the iLUX and the LipiFlow for the Treatment of Meibomian Gland Dysfunction and Symptoms: A Randomized Clinical Trial. Clin Ophthalmol. 2020;14:405-418. Published 2020 Feb 12.
23. Wesley G, Bickle K, Downing J, et al. Comparison of two thermal pulsation systems in the treatment of meibomian gland dysfunction: a randomized, multicenter study. Optom Vis Sci. 2022;99(4):323-332.
24. Schanzlin D, Owen JP, Klein S, et al. Efficacy of the Systane iLux thermal pulsation system for the treatment of meibomian gland dysfunction after 1 week and 1 month: a prospective study. Eye Contact Lens. 2022;48)4):155-161.
25. Gupta PK, Holland EJ, Hovanesian J, Loh J, Jackson MA, Karpecki PM, Dhamdhere K. TearCare for the Treatment of Meibomian Gland Dysfunction in Adult Patients With Dry Eye Disease: A Masked Randomized Controlled Trial. Cornea. 2022;41(4):417-426.
26. Sight Sciences Announces Presentation of Successful Phase I Results of the SAHARA Randomized Controlled Clinical Trial Comparing TearCare® to Restasis® for the Treatment of Dry Eye Disease at the American Academy of Optometry Annual Meeting [press release]. Sight Sciences; October 12, 2023; Menlo Park, CA.
27. Uchino M, Yokoi N, Shimazaki J, Hori Y, Tsubota K, On Behalf Of The Japan Dry Eye Society. Adherence to Eye Drops Usage in Dry Eye Patients and Reasons for Non-Compliance: A Web-Based Survey. J Clin Med. 2022;11(2):367.
28. Maskin SL. Intraductal meibomian gland probing relieves symptoms of obstructive meibomian gland dysfunction. Cornea. 2010;29(10):1145-1152.
29. Raulin C, Greve B, Grema H. IPL technology: a review. Lasers Surg Med. 2003;32(2):78-87.
30. Liu S, Tang S, Dong H, Huang X. Intense pulsed light for the treatment of Meibomian gland dysfunction: A systematic review and meta-analysis. Exp Ther Med. 2020;20(2):1815-1821.
31. Lumenis. Lumenis Receives FDA Approval for Its IPL Device to Manage Dry Eye Disease and Launches OptiLight. Published April 29, 2021. Accessed December 10, 2021. https://eyewire.news/news/lumenis-receives-fda-approval-for-its-ipl-device-to-manage-dry-eye-disease-and-launches-optilight.
32. Liu R, Rong B, Tu P, et al. Analysis of Cytokine Levels in Tears and Clinical Correlations After Intense Pulsed Light Treating Meibomian Gland Dysfunction. Am J Ophthalmol. 2017;183:81-90.
33. Yin Y, Liu N, Gong L, Song N. Changes in the Meibomian Gland After Exposure to Intense Pulsed Light in Meibomian Gland Dysfunction (MGD) Patients. Curr Eye Res. 2018;43(3):308-313.
34. Yan X, Hong J, Jin X, et al. The Efficacy of Intense Pulsed Light Combined With Meibomian Gland Expression for the Treatment of Dry Eye Disease Due to Meibomian Gland Dysfunction: A Multicenter, Randomized Controlled Trial. Eye Contact Lens. 2021;47(1):45-53.
35. Suwal A, Hao JL, Zhou DD, Liu XF, Suwal R, Lu CW. Use of Intense Pulsed Light to Mitigate Meibomian Gland Dysfunction for Dry Eye Disease. Int J Med Sci. 2020;17(10):1385-1392.
36. Azura Ophthalmics Announces Positive Results from Phase 2b Clinical Trial of AZR-MD-001 in Meibomian Gland Dysfunction [press release]. Azura Ophthalmics; November 17, 2022; Tel Aviv, Israel, and Melbourne, Australia.
37. Clark D, Sheppard J, Brady TC. A Randomized Double-Masked Phase 2a Trial to Evaluate Activity and Safety of Topical Ocular Reproxalap, a Novel RASP Inhibitor, in Dry Eye Disease. J Ocul Pharmacol Ther. 2021;37(4):193-199.
38. Aldeyra Therapeutics Announces Top-Line Results from the Phase 3 TRANQUILITY Trial in Dry Eye Disease [press release]. Aldeyra Therapeutics; December 20, 2021; Lexington, MA.
39. Aldeyra Therapeutics Achieves Primary Endpoint in Phase 3 TRANQUILITY2 Trial in Dry Eye Disease and Intends to Submit New Drug Application for Symptoms and Three Sign Endpoints of Dry Eye Disease [press release]. Aldeyra Therapeutics; June 8, 2022; Lexington, MA.
40. Aldeyra Therapeutics Receives Complete Response Letter from the U.S. Food and Drug Administration for the Reproxalap New Drug Application for the Treatment of Dry Eye Disease [press release]. Aldeyra Therapeutics; November 27, 2023; Lexington, MA.
Real-World Case From the Clinic
Walter O. Whitley, OD, MBA, FAAO
A 45-year-old white man presented for a second opinion on a dry eye disease (DED) evaluation. He reported bilateral blurred vision, tearing, and itching. He reported use of olopatadine for itching, as well as preservative-free artificial tears six times daily. His ocular history includes phacovitrectomy 4 years before presentation.
Upon completing a SPEED questionnaire, it was determined that the patient was symptomatic of DED with a score of 22 out of 28. BCVA was measured at 20/50 OD and 20/30 OS. Cloudy secretions from the meibomian glands were observed on slit lamp examination and moderate dropout was observed (Figure), although normal lid seal in both eyes was present. Diagnostic testing revealed tear breakup time (TBUT) of 3 seconds OD and 4 seconds OS, bilateral MMP-9, and tear osmolarity of 324 mOsms/L OD and 314 mOsms/L OS.
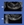 Figure. Examination of the lids in this patient showed moderate meibomian gland dropout in both eyes. The remaining meibomian glands produced cloudy secretions. Images courtesy Walter O. Whitley, OD, MBA, FAAO.
Figure. Examination of the lids in this patient showed moderate meibomian gland dropout in both eyes. The remaining meibomian glands produced cloudy secretions. Images courtesy Walter O. Whitley, OD, MBA, FAAO.The patient was diagnosed with keratoconjunctivitis sicca and bilateral meibomian gland dysfunction (MGD) involving both upper and lower lids. He was started on loteprednol etabonate ophthalmic suspension 0.25%, to be administered three times daily until the prescription was gone. He was directed to use a heat mask 5 minutes daily. Recommended over-the-counter interventions included nutraceutical supplementation and preservative-free lipid-based artificial tears. I discussed the possibility of thermal pulsation therapy with the patient, and directed him to return in 4 to 6 weeks for follow-up. I instructed my staff to not touch his eye upon return to the clinic so as to not interfere with staining tests upon return.
At follow-up, BCVA improved to 20/25 OU, and SPEED scoring had improved to 16/28 from 22/28. I attribute the improvement in both vision and SPEED scoring to patient compliance. He was negative for MMP-9 on follow-up. TBUT was measured at 4 seconds OU, and tear osmolarity improved to 307 mOsms/L OD and 312 mOsms/L OS. I directed the patient to exhaust his supply of loteprednol and to continue using a heat mask. Although I directed him to begin cyclosporine treatment, insurance mandated use of lifitegrast.
The patient returned in 3 months as directed, reporting that he did not like using lifitegrast due to blurring that occurred approximately 1 hour after application. The patient and I decided that thermal pulsation treatment would be appropriate moving forward.
At the time, perfluorohexyloctane ophthalmic solution was not approved by the FDA, so it was not an option. Had it been available, this patient would have been a good candidate for that intervention.















