Schizophrenia management has been based on D2 blockade for decades, with little to no advancements in the molecular mechanisms that drive schizophrenia or the drugs used to treat the disease. An exciting era where a move away from D2 blockade has recently begun and encompasses modulation of TAAR1 and M1/M4 muscarinic receptors to mitigate the adverse effects of current therapeutics as well as significantly advance schizophrenia management.
Schizophrenia (SCZ) is a highly heritable, severely disabling, chronic neurodevelopmental brain disorder that significantly impacts social functioning, work performance, and overall patient quality of life (QoL)1,2. The World Health Organization estimates that SCZ affects 1 in 300 people, with a global prevalence of approximately 24 million3. Despite having a relatively low prevalence, SCZ has an immense global impact on healthcare resources and QoL and is one of the leading causes of disability worldwide4. Therapeutic intervention for the management of SCZ within the first 5 years after the initial episode is crucial to stop or slow the progress of the positive and negative symptoms of SCZ as well as the pathophysiologic changes and neuronal remodeling occurring in the brain during this time5. The current paradigm in the treatment of SCZ has been focused primarily on D2 receptor blockade. Although current antipsychotics demonstrate efficacy against the positive symptoms of schizophrenia, they do not effectively treat the negative or cognitive symptom domains and are associated with substantial adverse effects, including movement disorders and metabolic side effects leading to increased cardiovascular risk6,7. The shortcomings of existing agents have led investigators to explore new pathways to develop new medications with novel mechanisms of action that are safer and more effective.
Newly Discovered Pathomechanisms that Drive Schizophrenia
Research suggests that a combination of physical, genetic, psychological, and environmental factors make an individual more susceptible to SCZ; however, some people are more prone to SCZ, where a stressful or emotional life event might trigger a psychotic episode8. Unfortunately, it is unknown why some individuals develop SCZ symptoms, and others do not. The complexity and heterogeneity of SCZ lend to the clinical challenges in treating SCZ. While the mechanisms that drive SCZ are intricate and relatively defined, the etiology of SCZ remains unclear1. The pathomechanisms of SCZ, once thought to be dopamine centric after decades of research, have been found to be extremely complicated and encompass numerous neuronal brain abnormalities and pathways.
With advances in academic and clinical research, the ideology of a single receptor to treat a multifaceted disease has shifted. Scientists have uncovered 3 emergent molecular pathways that offer new approaches to SCZ treatment management:
TAAR1 Receptors – Targeting the trace amine-associated receptor 1 (TAAR1) mechanism of action increases monoamines at the synaptic cleft of neurons. This increase induces the activation of G protein-coupled inwardly rectifying potassium channels, reducing synaptic firing of dopaminergic neurons, thus producing a hyperdopaminergic state9.
M1 and M4 Muscarinic Receptors - Muscarinic M1 and M4 receptors are expressed ubiquitously; however, activation of M1 and M4 receptors in the central nervous system regulates key dopaminergic and glutamatergic circuits in the brain that are believed to be imbalanced in patients suffering SCZ. Furthermore, cholinergic signaling through M1 and M4 receptors can robustly modulate circuitry involved in mediating positive, negative, and cognitive symptoms of SCZ10.
5-HT2A and 5-HT2C Receptors - Mechanisms of action that block the 5-HT2A and 5-HT2C receptors and are devoid of D2 receptor antagonism are being investigated. This pathway is critical in mediating because excessive stimulation of serotonergic receptors results in psychotic symptoms, including the visual hallucinations that are one of the hallmark symptoms of SCZ 11.
The modulation of these novel pathways offers clinicians and patients new therapeutic avenues that might manage SCZ symptoms more effectively, thus shifting the archaic D2 receptor treatment approach to a more global, multifaceted molecular paradigm.
New and Novel Treatment Approaches
The current paradigm in the treatment of SCZ has been focused primarily on D2 receptor blockade; however, due to more intelligently and rationally designed approaches, new pathways have been uncovered that allow for the development of new and novel SCZ therapies. Classically for decades, SCZ was thought to involve dopamine deficiencies or dysfunction; thus, therapies surrounding this neuronal circuit were the pharmacological target for the management of both positive and negative SCZ symptoms. As research progressed various neurotransmitters, such as glutamate, serotonin, and GABA (γ-aminobutyric acid), helped expand our current understanding of the pathophysiological drivers of SCZ12-14. Unfortunately, the use of typical and atypical antipsychotics leaves many symptoms unresolved, contributing to increased healthcare and personal cost burdens that arise from the significant cost-effect burden that D2 blockade causes15. While D2 blockade has been therapeutically beneficial, it also has created a significant unmet clinical need to find new, novel, safer, and more effective drugs that improve symptoms of SCZ and psychosis such as:
Ulotaront - a TAAR1 and 5-HT1A receptor agonist, has unique mechanisms of action; while not a specific D2 receptor inhibitor, it has been shown to facilitate the internalization of D2 receptors; therefore, less D2 receptor occupancy is achieved without actually blocking the receptor9. Additionally, a reduction in presynaptic dopamine firing was observed, reducing excess striatal dopamine synthesis, one of the actual mechanisms that underlies the pathophysiology of SCZ9. A phase 2 clinical trial revealed that ulotaront administered for 4 weeks at 2 doses of 50 and 75 mg per day was associated with significant reductions in PANSS (Positive and Negative Syndrome Scale), CGI-S (Clinical Global Impressions Scale), and all PANSS subscale scores compared to placebo16.
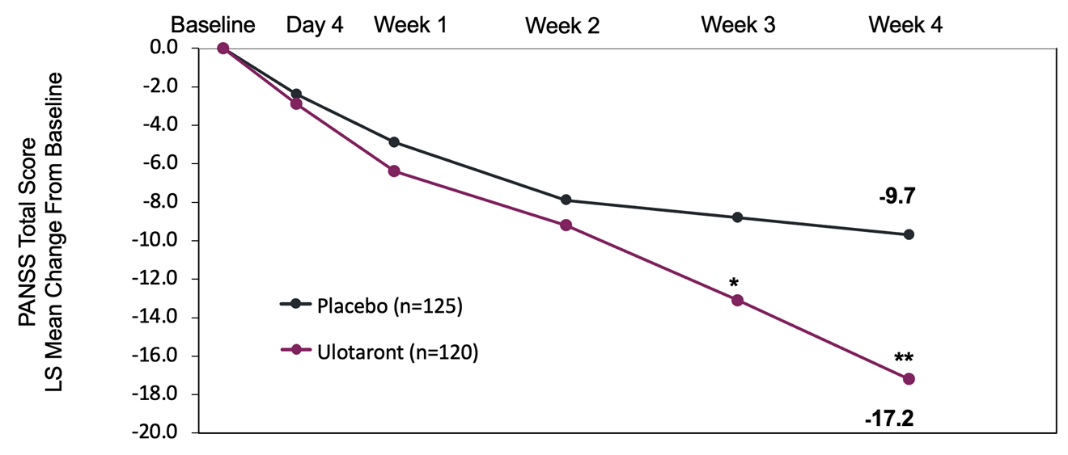
Figure 1 - Ulotaront Study 201 Primary Endpoint: PANSS Total Score
Xanomeline - a muscarinic cholinergic receptor agonist that binds to M1 and M4 muscarinic receptors17. During development, xanomeline was found to bind the M4 receptors in the brain, reducing acetylcholine release, and in turn reducing dopamine transmission17,18. Like ulotaront, xanomeline reduces dopamine transmission and firing without blocking the D2 dopamine receptor. Unfortunately, during its investigation significant cholinergic side effects were observed and caused a cessation of the drug trials despite promising results19. Xanomeline was reformulated with the addition of trospium, a noncentrally active noncholinergic drug, to mitigate the side effects that caused the clinical trials to stop. During the phase 2B trials, participants were administered either xanomeline 50 mg combined with 20 mg trospium twice a day or were increased to 125 mg xanomeline combined with 30 mg trospium twice a day20. After 5 weeks, the participants of the study showed significant improvements in the reduction of PANSS total and subscale scores.
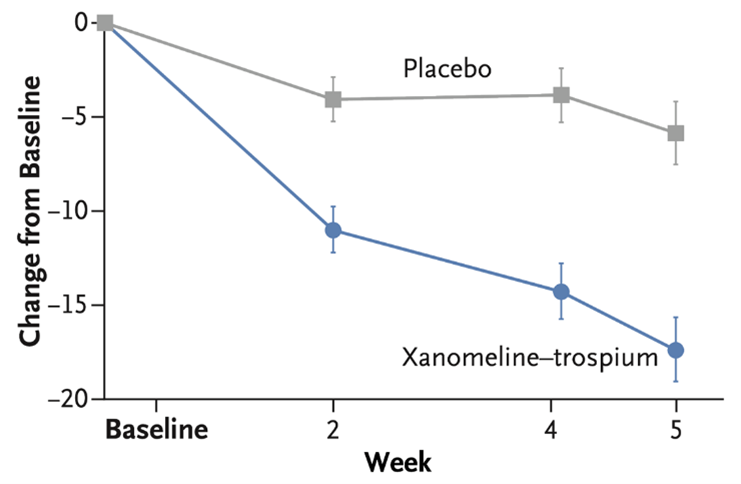
Figure 2 -Xanomeline-Trospium Phase 2b Results: PANSS Total Score
Newer, safer, and more efficacious drugs to treat SCZ are long overdue and severely needed to fill the clinical gaps of care for SCZ. The lack of knowledge surrounding the multifaceted cellular and molecular pathways that drive SCZ pathophysiology make drug target advancement challenging; however, new scientific evidence and a deeper understanding of the mechanisms that underlie the pathomechanisms of SCZ allow for the advances in drug design needed to shift the current SCZ treatment paradigm from a dopaminergic-centric approach to a more global neurotransmitter understanding of SCZ. This shift will allow for significant improvements in patient outcomes and QoL and potentially close the one gap that D2 receptor blockade has yet to achieve, that of mortality.
References
- McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia-an overview. JAMA Psychiatry. 2020:77(2):201-210.
- National Institute of Mental Health (NIMH). Schizophrenia. https://www.nimh.nih.gov/health/statistics/schizophrenia.
- World Health Organization (WHO). Schizophrenia. January 10, 2022. https://www.who.int/news-room/fact-sheets/detail/schizophrenia.
- Chong HY, Teoh SL, Wu DB, et al. Global economic burden of schizophrenia: a systematic review. Neuropsychiatr Dis Treat. 2016;12:357-373.
- National Institute of Mental Health (NIMH). Recovery after an initial schizophrenia episode (RAISE). October 2022. https://www.nimh.nih.gov/research/research-funded-by-nimh/research-initiatives/recovery-after-an-initial-schizophrenia-episode-raise.
- Meltzer HY. Update on typical and atypical antipsychotic drugs. Annu Rev Med. 2013;64:393-406.
- Meltzer HY, Gadaleta E. Contrasting typical and atypical antipsychotic drugs. Focus. 2021;19(1):3-13.
- National Health Service (NHS). Causes-schizophrenia. 2021. https://www.nhs.uk/mental-health/conditions/schizophrenia/causes.
- TAAR1 agonists - an overview. ScienceDirect Topics. https://www.sciencedirect.com/topics/neuroscience/taar1-agonists.
- Foster DJ, Bryant ZK, Conn PJ. Targeting muscarinic receptors to treat schizophrenia. Behav Brain Res. 2021;405:113201.
- Capuzzi E, Caldiroli A, Ciscato V, et al. Experimental serotonergic agents for the treatment of schizophrenia. J Exp Pharmacol. 2021;13,49-67.
- Carlsson A, Waters N, Holm-Waters S, et al. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001;41:237-260.
- Egerton A, Grace AA, Stone J, et al. Glutamate in schizophrenia: neurodevelopmental perspectives and drug development. Schizophr Res. 2020;223:59-70.
- Yang AC, Tsai SJ. New targets for schizophrenia treatment beyond the dopamine hypothesis. Int J Mol Sci. 2017;18(8):1689.
- Wright P, O'Flaherty L. Antipsychotic drugs: atypical advantages and typical disadvantages. Ir J Psychol Med. 2003;20(1):24-27.
- Correll CU, Koblan KS, Hopkins SC, et al. Safety and effectiveness of ulotaront (SEP-363856) in schizophrenia: results of a 6-month, open-label extension study. NPJ Schizophr. 2021;7(1):63.
- Powers, A. S. et al. P598. Exploring the molecular determinants for functional selectivity of the antipsychotic xanomeline at muscarinic acetylcholine receptors. Biol Psychiatry. 2022;91(9):S331.
- Bridges TM, LeBois EP, Hopkins CR, et al. The antipsychotic potential of muscarinic allosteric modulation. Drug News Perspect. 2010;23(4):229-240.
- Brannan SK, Sawchak S, Miller AC, et al. Muscarinic cholinergic receptor agonist and peripheral antagonist for schizophrenia. N Engl J Med. 2021;384(8):717-726.
- Weiden PJ, Breier A, Kavanagh S, et al. Antipsychotic efficacy of KarXT (xanomeline-trospium): post hoc analysis of Positive and Negative Syndrome Scale categorical response rates, time course of response, and symptom domains of response in a phase 2 study. J Clin Psychiatry. 2022;83(3):21m14316.




Facebook Comments