ReachMD
Be part of the knowledge.™Serelaxine: New treatment acute heart failure?
Literature -
J Am Coll Cardiol. 2013 Jan 15;61(2):196-206. doi: 10.1016/j.jacc.2012.11.005.
Lancet. 2013 Jan 5;381(9860):29-39. doi: 10.1016/S0140-6736(12)61855-8.
The RELAX-AHF-1 study assessed the efficacy and safety of serelaxin compared with placebo in patients with acute heart failure. Serelaxin is a peptide hormone or a chain of molecules, dilating blood vessels and improving the blood flow to organs, probably protecting them against acute damage.
The RELAX-AHF study included 1160 patients with AHF and systolic blood pressure >125 mm Hg randomized to treatment with serelaxin via a 48-hour intravenous infusion within 16 hours of presentation or placebo. The dose of serelaxin, 30 µg/kg per day, was selected based on a phase 2 dose-ranging study.
1. Fonarow GC, Abraham WT, Albert NM, et al. Influence of a performance-improvement initiative on quality of care for patients hospitalized with heart failure: results of the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF). Arch Intern Med 2007;167:1493–502.
2. Solomon SD, Dobson J, Pocock S, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation 2007;116:1482–7.
3. Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol 2009;53:557–73.
4. McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787– 847.
5. Laribi S, Aouba A, Nikolaou M, et al. Trends in death attributed to heart failure over the past two decades in Europe. Eur J Heart Fail 2012;14:234 –9.
6. Mebazaa A, Pang PS, Tavares M, et al. The impact of early standard therapy on dyspnoea in patients with acute heart failure: the URGENT-dyspnoea study. Eur Heart J 2010; 31: 832–41.
7. Metra M, O’Connor CM, Davison BA, et al. Early dyspnoea relief in acute heart failure: prevalence, association with mortality, and effect of rolofylline in the PROTECT Study. Eur Heart J 2011; 32: 1519–34.
8. Metra M, Teerlink JR, Felker GM, et al. Dyspnoea and worsening heart failure in patients with acute heart failure: results from the Pre-RELAX-AHF study. Eur J Heart Fail 2010; 12: 1130–39.
9. Gheorghiade M, Konstam MA, Burnett JC Jr, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA 2007; 297: 1332–43.
10. McMurray JJ, Teerlink JR, Cotter G, et al. Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials. JAMA 2007; 298: 2009–19.
11. O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011; 365: 32–43.
The aim of this study was to assess the effects of serelaxin on short-term changes in markers of organ damage and congestion and relate them to 180-day mortality in patients with acute heart failure.
Background: Hospitalization for acute heart failure is associated with high post-discharge mortality, and this may be related to organ damage.
Methods:
The Pre-RELAX-AHF (Relaxin in Acute Heart Failure) phase II study and RELAX-AHF phase III study were international, multicenter, double-blind, placebo-controlled trials in which patients hospitalized for acute heart failure were randomized within 16 h to intravenous placebo or serelaxin. Each patient was followed daily to day 5 or discharge and at days 5, 14, and 60 after enrollment. Vital status was assessed through 180 days. In RELAX-AHF, laboratory evaluations were performed daily to day 5 and at day 14. Plasma levels of biomarkers were measured at baseline and days 2, 5, and 14. All-cause mortality was assessed as a safety endpoint in both studies.
Results:
Serelaxin reduced 180-day mortality, with similar effects in the phase II and phase III studies (combined studies: N = 1,395; hazard ratio: 0.62; 95% confidence interval: 0.43 to 0.88; p = 0.0076). In RELAX-AHF, changes in markers of cardiac (high-sensitivity cardiac troponin T), renal (creatinine and cystatin-C), and hepatic (aspartate transaminase and alanine transaminase) damage and of decongestion (N-terminal pro-brain natriuretic peptide) at day 2 and worsening heart failure during admission were associated with 180-day mortality. Serelaxin administration improved these markers, consistent with the prevention of organ damage and faster decongestion.
Conclusions:
Early administration of serelaxin was associated with a reduction of 180-day mortality, and this occurred with fewer signs of organ damage and more rapid relief of congestion during the first days after admission.
Serelaxin, recombinant human relaxin-2, is a vasoactive peptide hormone with many biological and haemodynamic effects. In a pilot study, serelaxin was safe and well tolerated with positive clinical outcome signals in patients with acute heart failure. The RELAX-AHF trial tested the hypothesis that serelaxin-treated patients would have greater dyspnoea relief compared with patients treated with standard care and placebo.
Methods:
RELAX-AHF was an international, double-blind, placebo-controlled trial, enrolling patients admitted to hospital for acute heart failure who were randomly assigned (1:1) via a central randomisation scheme blocked by study centre to standard care plus 48-h intravenous infusions of placebo or serelaxin (30 μg/kg per day) within 16 h from presentation. All patients had dyspnoea, congestion on chest radiograph, increased brain natriuretic peptide (BNP) or N-terminal prohormone of BNP, mild-to-moderate renal insufficiency, and systolic blood pressure greater than 125 mm Hg. Patients, personnel administering study drug, and those undertaking study-related assessments were masked to treatment assignment. The primary endpoints evaluating dyspnoea improvement were change from baseline in the visual analogue scale area under the curve (VAS AUC) to day 5 and the proportion of patients with moderate or marked dyspnoea improvement measured by Likert scale during the first 24 h, both analysed by intention to treat. This trial is registered at ClinicalTrials.gov, NCT00520806.
Findings:
1161 patients were randomly assigned to serelaxin (n=581) or placebo (n=580). Serelaxin improved the VAS AUC primary dyspnoea endpoint (448 mm × h, 95% CI 120-775; p=0•007) compared with placebo, but had no significant effect on the other primary endpoint (Likert scale; placebo, 150 patients [26%]; serelaxin, 156 [27%]; p=0•70). No significant effects were recorded for the secondary endpoints of cardiovascular death or readmission to hospital for heart failure or renal failure (placebo, 75 events [60-day Kaplan-Meier estimate, 13•0%]; serelaxin, 76 events [13•2%]; hazard ratio [HR] 1•02 [0•74-1•41], p=0•89] or days alive out of the hospital up to day 60 (placebo, 47•7 [SD 12•1] days; serelaxin, 48•3 [11•6]; p=0•37). Serelaxin treatment was associated with significant reductions of other prespecified additional endpoints, including fewer deaths at day 180 (placebo, 65 deaths; serelaxin, 42; HR 0•63, 95% CI 0•42-0•93; p=0•019).
Interpretation:
Treatment of acute heart failure with serelaxin was associated with dyspnoea relief and improvement in other clinical outcomes, but had no effect on readmission to hospital. Serelaxin treatment was well tolerated and
1. Effect of Serelaxin on Cardiac, Renal, and Hepatic Biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) Development Program: Correlation With Outcomes.
Metra M, Cotter G, Davison BA, et al; RELAX-AHF Investigators.J Am Coll Cardiol. 2013 Jan 15;61(2):196-206. doi: 10.1016/j.jacc.2012.11.005.
2. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial.
Teerlink JR, Cotter G, Davison BA, et al; RELAXin in Acute Heart Failure (RELAX-AHF) Investigators.Lancet. 2013 Jan 5;381(9860):29-39. doi: 10.1016/S0140-6736(12)61855-8.
Background
Acute heart failure is the most frequent cause of hospitalization in patients older than 65 years, with a mortality rate of 30-40% within 1 year [1-5]. Despite a favourable response to initial treatment, most patients remain symptomatic at 24 h and up to 25% develop worsening symptoms during the hospital stay [6-8]. Sustained relief of these signs and symptoms remains an important goal of treatment [9-11].The RELAX-AHF-1 study assessed the efficacy and safety of serelaxin compared with placebo in patients with acute heart failure. Serelaxin is a peptide hormone or a chain of molecules, dilating blood vessels and improving the blood flow to organs, probably protecting them against acute damage.
The RELAX-AHF study included 1160 patients with AHF and systolic blood pressure >125 mm Hg randomized to treatment with serelaxin via a 48-hour intravenous infusion within 16 hours of presentation or placebo. The dose of serelaxin, 30 µg/kg per day, was selected based on a phase 2 dose-ranging study.
Results:
- Compared with placebo, patients receiving serelaxin a significant reduction was observed in symptoms of heart failure with a 20% reduction in the degree of dyspnea. Moreover patients who received serelaxin had:
- more than 45% fewer episodes of worsening heart failure symptoms during hospitalization;
- almost half a day less time spent in the intensive care units;
- almost a full day shorter hospital stay.
- Serelaxin did not reduce the number of rehospitalisation in patients with heart failure.
- Serelaxin caused improvement in markers of cardiac, renal and liver damage and persistent congestion during the first few days after admission, associated with improved 180-day mortality.
Conclusion:
Serelaxine is promising as the first evidence-based therapy for acute heart failure which can significantly improve symptoms and clinical outcomes, including death.The significant reductions in exacerbation of heart failure and mortality are encouraging signs that the course of this devastating disease can be changed.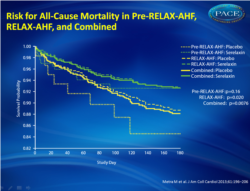 Figure 1Risk for All-Cause Mortality in Pre-RELAX-AHF, RELAX-AHF, and Combined .The combined results represent stratified Kaplan-Meier estimates. |
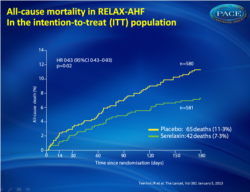 Figure 2All-cause death during 180 days of follow-up in the placebo-treated group compared with the group that received serelaxin in the intention-to-treat (ITT) population |
References
1. Fonarow GC, Abraham WT, Albert NM, et al. Influence of a performance-improvement initiative on quality of care for patients hospitalized with heart failure: results of the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF). Arch Intern Med 2007;167:1493–502.2. Solomon SD, Dobson J, Pocock S, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation 2007;116:1482–7.
3. Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol 2009;53:557–73.
4. McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787– 847.
5. Laribi S, Aouba A, Nikolaou M, et al. Trends in death attributed to heart failure over the past two decades in Europe. Eur J Heart Fail 2012;14:234 –9.
6. Mebazaa A, Pang PS, Tavares M, et al. The impact of early standard therapy on dyspnoea in patients with acute heart failure: the URGENT-dyspnoea study. Eur Heart J 2010; 31: 832–41.
7. Metra M, O’Connor CM, Davison BA, et al. Early dyspnoea relief in acute heart failure: prevalence, association with mortality, and effect of rolofylline in the PROTECT Study. Eur Heart J 2011; 32: 1519–34.
8. Metra M, Teerlink JR, Felker GM, et al. Dyspnoea and worsening heart failure in patients with acute heart failure: results from the Pre-RELAX-AHF study. Eur J Heart Fail 2010; 12: 1130–39.
9. Gheorghiade M, Konstam MA, Burnett JC Jr, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA 2007; 297: 1332–43.
10. McMurray JJ, Teerlink JR, Cotter G, et al. Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials. JAMA 2007; 298: 2009–19.
11. O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011; 365: 32–43.
Abstract (1)
Objectives:The aim of this study was to assess the effects of serelaxin on short-term changes in markers of organ damage and congestion and relate them to 180-day mortality in patients with acute heart failure.
Background: Hospitalization for acute heart failure is associated with high post-discharge mortality, and this may be related to organ damage.
Methods:
The Pre-RELAX-AHF (Relaxin in Acute Heart Failure) phase II study and RELAX-AHF phase III study were international, multicenter, double-blind, placebo-controlled trials in which patients hospitalized for acute heart failure were randomized within 16 h to intravenous placebo or serelaxin. Each patient was followed daily to day 5 or discharge and at days 5, 14, and 60 after enrollment. Vital status was assessed through 180 days. In RELAX-AHF, laboratory evaluations were performed daily to day 5 and at day 14. Plasma levels of biomarkers were measured at baseline and days 2, 5, and 14. All-cause mortality was assessed as a safety endpoint in both studies.
Results:
Serelaxin reduced 180-day mortality, with similar effects in the phase II and phase III studies (combined studies: N = 1,395; hazard ratio: 0.62; 95% confidence interval: 0.43 to 0.88; p = 0.0076). In RELAX-AHF, changes in markers of cardiac (high-sensitivity cardiac troponin T), renal (creatinine and cystatin-C), and hepatic (aspartate transaminase and alanine transaminase) damage and of decongestion (N-terminal pro-brain natriuretic peptide) at day 2 and worsening heart failure during admission were associated with 180-day mortality. Serelaxin administration improved these markers, consistent with the prevention of organ damage and faster decongestion.
Conclusions:
Early administration of serelaxin was associated with a reduction of 180-day mortality, and this occurred with fewer signs of organ damage and more rapid relief of congestion during the first days after admission.
Abstract (2)
Background:Serelaxin, recombinant human relaxin-2, is a vasoactive peptide hormone with many biological and haemodynamic effects. In a pilot study, serelaxin was safe and well tolerated with positive clinical outcome signals in patients with acute heart failure. The RELAX-AHF trial tested the hypothesis that serelaxin-treated patients would have greater dyspnoea relief compared with patients treated with standard care and placebo.
Methods:
RELAX-AHF was an international, double-blind, placebo-controlled trial, enrolling patients admitted to hospital for acute heart failure who were randomly assigned (1:1) via a central randomisation scheme blocked by study centre to standard care plus 48-h intravenous infusions of placebo or serelaxin (30 μg/kg per day) within 16 h from presentation. All patients had dyspnoea, congestion on chest radiograph, increased brain natriuretic peptide (BNP) or N-terminal prohormone of BNP, mild-to-moderate renal insufficiency, and systolic blood pressure greater than 125 mm Hg. Patients, personnel administering study drug, and those undertaking study-related assessments were masked to treatment assignment. The primary endpoints evaluating dyspnoea improvement were change from baseline in the visual analogue scale area under the curve (VAS AUC) to day 5 and the proportion of patients with moderate or marked dyspnoea improvement measured by Likert scale during the first 24 h, both analysed by intention to treat. This trial is registered at ClinicalTrials.gov, NCT00520806.
Findings:
1161 patients were randomly assigned to serelaxin (n=581) or placebo (n=580). Serelaxin improved the VAS AUC primary dyspnoea endpoint (448 mm × h, 95% CI 120-775; p=0•007) compared with placebo, but had no significant effect on the other primary endpoint (Likert scale; placebo, 150 patients [26%]; serelaxin, 156 [27%]; p=0•70). No significant effects were recorded for the secondary endpoints of cardiovascular death or readmission to hospital for heart failure or renal failure (placebo, 75 events [60-day Kaplan-Meier estimate, 13•0%]; serelaxin, 76 events [13•2%]; hazard ratio [HR] 1•02 [0•74-1•41], p=0•89] or days alive out of the hospital up to day 60 (placebo, 47•7 [SD 12•1] days; serelaxin, 48•3 [11•6]; p=0•37). Serelaxin treatment was associated with significant reductions of other prespecified additional endpoints, including fewer deaths at day 180 (placebo, 65 deaths; serelaxin, 42; HR 0•63, 95% CI 0•42-0•93; p=0•019).
Interpretation:
Treatment of acute heart failure with serelaxin was associated with dyspnoea relief and improvement in other clinical outcomes, but had no effect on readmission to hospital. Serelaxin treatment was well tolerated and
Facebook Comments