ReachMD
Be part of the knowledge.™Gut microbe-dependent metabolite levels have prognostic value in heart failure
Tang WHW, Wang Z, Fan Y et al.,
J Am Coll Cardiol. 2014;64(18):1908-1914. doi:10.1016/j.jacc.2014.02.617
Background
The contribution of the gastrointestinal system to the pathogenesis of heart failure (HF) is increasingly appreciated [1,2]. A well-balanced intestinal microbial community normally maintains intestinal barrier function, along with mucosal immunity and normal sodium and water homeostasis. In HF and associated splanchnic circulation congestion, altered intestinal barrier function, bacterial overgrowth and impaired host defence can disturb the bacterial environment. Ultimately this may contribute to systemic inflammation [3,4]. Progressive venous congestion in some patients may further complicate abdominal congestion, including negatively affecting drug absorption and pharmacokinetics [5,6], and renal glomerular and tubular dysfunction due to increased intra-abdominal pressure [7,8].A mechanistic link has previously been described between intestinal microbe-dependent generation of trimethylamine-N-oxide (TMAO) and increased risk for future cardiovascular events, via a pathway involving dietary nutrients such as phosphatidylcholine, choline (found in eggs) and carnitine (found in red meat) [9-11]. The authors investigated the relationship between fasting plasma TMAO levels and long-term (5 years) clinical prognosis in patients with stable HF.
Main results
- Mean TMAO level in the HF cohort was 5.0 µM (IQR: 3.0-8.5 µM), as compared to 3.5 µM (IQR: 2.3-5.7) in the healthy cohort (P<0.001).
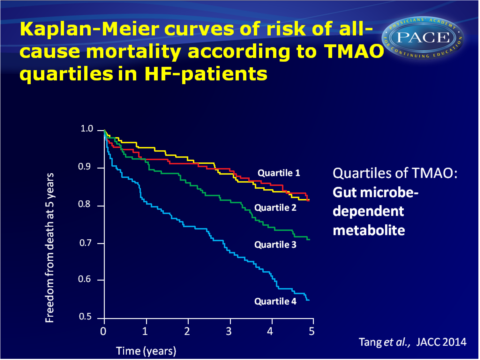
- Kaplan-Meier analysis of TMAO stratified by quartiles shows a graded increased mortality risk especially when TMAO levels are higher than median levels. Within the HF cohort, increased mortality was seen in the 4th TMAO quartile as compared with the 1st quartile (HR: 3.42, 95%CI: 2.24-5.23, P<0.001).
- Mortality risks associated with TMAO levels were similar in ischaemic and nonischaemic patients with HF.
- Addition of TMAO to a risk prediction model with traditional CV risk factors significantly improved net reclassification (integrated discrimination improvement: 16.0%, P<0.001, net reclassification index: 10.9%, P<0.001).
- A significant but modest correlation was seen between TMAO and BNP levels (r-0.23, P<0.001). The correlation between TMAO and eGFR was stronger (r= -0.55, P<0.001).
- Even in a model that corrected for traditional risk factors, BNP, eGRF and hsCRP, elevated TMAO levels were associated with increased mortality risk (HR: 1.85, 95%CI: 1.14-3.00, P<0.05).
Download TANG JACC 2014 pace.pptx
Conclusion
This study shows that plasma TMAO has a strong prognostic value in patients with stable HF, in addition to traditional risk factors, cardiorenal indexes (BNP and eGFR) and a marker of systemic inflammation (hsCRP). These observations further define the ‘gut hypothesis’, in that gut microbiota affect prognosis in patients with HF. Studies are warranted that explore whether targeted interventions to alter the gut microbiota composition can lower TMAO levels and limit disease progression in HF.Editorial comment [12]
“The strong correlation between TMAO concentration and kidney function raises the following question: given the importance of the kidney in eliminating TMAO, is higher TMAO level just a markerof renal impairment? Other questions arise—for example, what is the role of comorbidities, such as diabetes, in elevating TMAO levels? Diabetes was considerably more common in the higher TMAO group, and metformin has been reported to increase TMAO levels (and intestinal microbiota have been postulated to play a role in the development of diabetes). More puzzling is just how TMAO by itself might relate to prognosis. It is probably unlikely that a proatherogenic mechanism could account for an increase in mortality in such a short time frame, especially in patients with nonobstructive coronary disease. (…) Clearly, this is only the beginning of the story of TMAO in HF
but one for which we should look forward to further instalments.”
Find this article online
References
1. Krack A, Sharma R, Figulla HR, Anker SD. The importance of the gastrointestinal system in the pathogenesis of heart failure. Eur Heart J 2005; 26:2368–74.
2. Verbrugge FH, Dupont M, Steels P, et al. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol 2013;62:485–95.
3. Rauchhaus M, Anker SD. Plasma concentrations of bacterial lipopolysaccharide: a marker of infection or inflammation? J Am Coll Cardiol 2000;36:656–7.
4. Sandek A, Bauditz J, Swidsinski A, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol 2007;50:1561–9.
5. Vasko MR, Cartwright DB, Knochel JP, et al. Furosemide absorption altered in decompensated congestive heart failure. Ann Intern Med 1985;102:314–8.
6. Carlton LD, Pollack GM, Brouwer KL. Physiologic pharmacokinetic modeling of gastrointestinal blood flow as a rate-limiting step in the oral absorption of digoxin: implications for patients with congestive heart failure receiving epoprostenol. J Pharm Sci 1996;85:473–7.
7. Mullens W, Abrahams Z, Skouri HN, et al. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol
2008;51:300–6.
8. Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009;53: 589–96.
9. Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63.
10. Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368: 1575–84.
11. Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–85.
12. Cannon JA, McMurray JJV. Gut feelings about heart failure. J Am Coll Cardiol 2014;64;18:1915-1916
Facebook Comments