ReachMD
Be part of the knowledge.™Favorable Skin Tolerability and Adhesion Results Found in Study of Adlarity Transdermal Patches
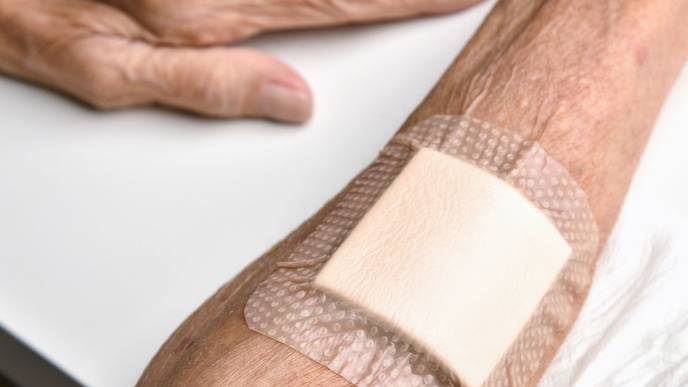
Results of a study published online in Alzheimer Disease & Associated Disorders revealed favorable outcomes in terms of skin tolerability and adhesion for Adlarity (donepezil transdermal system; Corium, Boston, MA) compared with placebo. The Food and Drug Administration (FDA) approved Adlarity (an acetylcholinesterase inhibitor) in 2022 as a treatment for people with Alzheimer disease (AD) who have mild, moderate, and severe dementia.
The double-blind phase 1 study (NCT03397862) enrolled 256 healthy adult participants aged 40 years and older. Study investigators administered an Adlarity skin patch and a placebo skin patch to alternate sides of each participants’ backs. The patches were replaced each week for 3 weeks, with participants receiving the new Adlarity and placebo patches at the same locations on their back each time. It’s important to note that this study procedure differs from the prescribed Adlarity administration instructions, which state that the patch should not return to a given location until 2 weeks have elapsed since removal. Study investigators assessed skin irritation at days 8, 15, and 22 using a combined skin irritation score (CSIS) averaged from measured Dermal Response scores and Other Effects scores. After 3 weeks, skin irritation scores showed minimal irritation and sensitization in both areas assessed: skin covered by an Adlarity patch or a placebo patch. Only 7 of the 735 Adlarity patches administered in the study detached, demonstrating good adhesion. Four of the participants showed potential skin sensitization to the Adlarity patches. Ten participants discontinued the study due to mildly increased blood pressure (>140 mmHg systolic) while 1 discontinued due to application-site reactions and edema. Common treatment-emergent adverse events (TEAEs) included application-site pruritis, headache, nausea, abnormal dreams, muscle spasms, and insomnia.
"The results grow our understanding of ADLARITY's safety profile and provide additional information that should be helpful to clinicians when considering the only weekly skin patch formulation of donepezil for their Alzheimer's patients," said Charles Oh, MD, Chief Medical Officer of Corium.
The active ingredient of Adlarity is donepezil, which is the most prescribed dementia medication in the United States.
Facebook Comments