Announcer Open:
Welcome to CME on ReachMD. This activity, titled Improving Outcomes and Addressing Racial Disparities in Patients With HR+/HER2- Early Breast Cancer: A Case-Based Learning Lab is developed by AXIS Medical Education and is supported by an educational grant from Novartis Pharmaceuticals Corporation.
Before starting this activity, please be sure to review the disclosure statements as well as the learning objectives.
Dr. Tolaney:
Hello and welcome to this educational activity.
My name is Sarah Tolaney. I am Chief of the Division of Breast Oncology and Associate Director of the Susan Smith Center for Women's Cancers at Dana-Farber Cancer Institute, and also Associate Professor of Medicine at Harvard Medical School. Today we'll be reviewing a patient case study to discuss the application and selection of CDK4/6 inhibitors for high-risk hormone receptor positive early breast cancer patients. So, let's go ahead and dive in and begin.
So, this is a case of a 48-year-old premenopausal black woman who had palpated a mass in her right breast. She underwent imaging and was found to have a 3-and-a-half-centimeter mass and a biopsy of this area demonstrated a grade 2 invasive lobular carcinoma. It was estrogen receptor strongly positive at 95%, progesterone receptor positive also 95%, and HER2 was 1 plus. She also had an enlarged axillary node on the ipsilateral side and an axillary ultrasound was performed and an FNA was done of that lymph node, which was positive for malignant cells. She underwent upfront surgery and was found to have a 4.1-centimeter grade 2 invasive lobular carcinoma with 2 of 7 lymph nodes that were involved. So, this brings us to a question: What would be your next step for this patient?
So certainly, there are lots of different tests we can consider in this setting. I think one of the tests that I think many of us would probably want to get in this setting is to get an Oncotype test in order to understand if this person would benefit from adjuvant systemic therapy. We do have data from the RxPONDER study in patients who did have 1 to 3 positive nodes and had hormone receptor-positive breast cancer that really showed us that, particularly in postmenopausal women, if you had a score of 25 or less, you really weren't benefiting from adjuvant chemotherapy. So, this to me is quite critical in patients who have early-stage breast cancer that is hormone receptor-positive for us to obtain in order to know who really needs chemotherapy.
There are also other biomarkers we could consider like Ki-67. This is really a marker trying to understand proliferation of the cancer, and there is data to suggest that having a higher Ki-67 is associated with higher risk of recurrence, so it is a prognostic marker. And while initially when we saw approval for a CDK4/6 inhibitor, it did come with needing to have a high Ki-67 for its indication. That has since been removed. So, in fact, one does not need to have information regarding Ki-67 to make a decision about use of adjuvant CDK4/6 inhibition.
And so, in this particular patient, the thing that I would have really wanted to know would have been an Oncotype test to make sure if I needed chemotherapy or not in that particular patient.
When thinking about risk in this patient, I think there are lots of factors that we have to consider. One is nodal positivity. We certainly know that the more lymph nodes that someone has involved, is associated with a higher risk of recurrence. The other thing we do consider, that is an independent prognostic indicator, is grade. So, particularly having a high-grade tumor, so grade 3 cancers, are also associated with higher risk of recurrence. The third important factor in my mind is tumor size. So again, the larger the tumor, the higher the risk. So, I think the 3 major points that I carry in my head when just looking at clinical anatomic information is grade, size, and nodal involvement.
There's certainly lots of other factors that are associated with higher risk – being of younger age, having positive margins – those are things that we also certainly take into account. And so, when we're making a decision in someone in the adjuvant setting who has early-stage hormone receptor-positive breast cancer, and we want to understand which patients could be candidates for abemaciclib, the major factors you have to think about are how many lymph nodes are involved. So, if someone has 4 or more positive lymph nodes, they are a candidate for abemaciclib. If they have 1 to 3 positive nodes, they are a candidate if they have a high-grade cancer or they have a tumor that is 5 centimeters or larger.
So unfortunately, approximately 20 to 30% of all patients who have early breast cancer will experience a relapse. And when we're trying to understand what are the factors that are associated with higher risk or recurrence, we've already mentioned that clinically, anatomic risk is something we do need to factor in. So, we need to know grade, nodal status, tumor size. But there are also other factors that we can consider. So, being of younger age is associated with higher risk of recurrence, having PR negativity, having higher proliferation scores, having evidence of lymphovascular invasion. And then, certain subtypes of breast cancer, for example, also are associated with higher risk, such as metaplastic carcinomas.
So, all of these things, again, come into play when trying to understand risk for an individual patient. And so, when we turn back to our particular patient, this patient did end up getting an Oncotype DX score and it did come back at 11. And so, if we remember from our data from RxPONDER that patients who had scores under 25 generally did not benefit from chemotherapy. However, there was data to suggest that pre-menopausal patients still were deriving some benefit from chemotherapy, and that benefit, again, was seen across scores that were less than 25. And so, it does make it complex when making a decision for a premenopausal patient, but I think in this case, because the patient had a lobular carcinoma and had a particularly low Oncotype DX, so coming back at 11, and discussing preferences with the patient, obviously very critical. And this patient was trying to avoid chemotherapy and so she elected not to get adjuvant chemotherapy and wanted to focus on maximizing endocrine therapy. So, in this case, went on to get ovarian suppression and an aromatase inhibitor. So, very critical in these cases where the decision is not so clear in my mind, when someone's premenopausal and has a recurrence score under 25, about whether or not we really need the chemotherapy or not, to really make a shared decision with your patient to really make sure that the patient’s values are getting factored into this decision.
So, now the question is, if this patient has gone on to ovarian suppression and aromatase inhibitor, would you add a CDK4/6 inhibitor? And, if you do decide to use a CDK4/6 inhibitor, which one would you use?
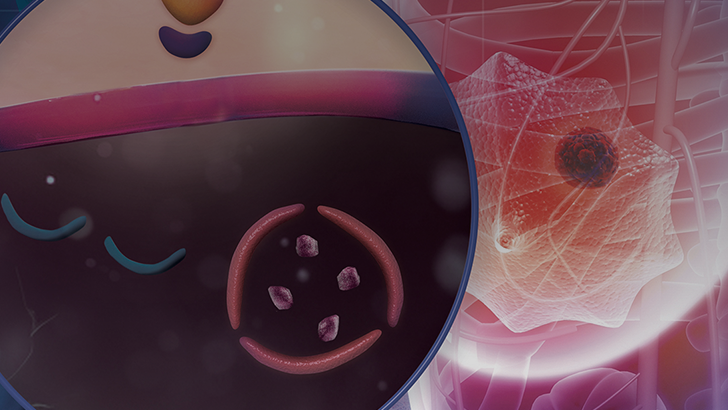
So, when we think back to CDK4/6 inhibition and remember how these agents actually work, CDK4/6 in the pathway of breast cancer works by phosphorylating the retinoblastoma protein. This causes release of E2F, and then this causes a transition in the cell cycle from the G1 to S phase. So really, this is keeping the cell cycle going. But if you inhibit CDK4/6, in essence you halt the cell cycle at that G1/S transition point, and you put the cell into a senescent state. And there's some thought that this senescence can lead to eventual apoptosis.
And so, there are 3 different CDK4/6 inhibitors that are approved in the metastatic setting: palbociclib, abemaciclib, and ribociclib. But at this point, right now, we have approval just for abemaciclib in the early disease setting, but I think we anticipate that ribociclib should soon be approved. There have been many studies that have tried to look at adding a CDK4/6 inhibitor in the adjuvant space. Some of the first studies that were done actually looked at adding palbociclib to endocrine therapy in the early disease setting. So, there were 2 studies that looked at palbociclib. Unfortunately, both of these studies were negative trials, meaning that adding palbociclib to endocrine therapy did not improve long-term outcomes. However, we have seen two other trials that have been positive.
The first of these is the monarchE study. This trial specifically looked at giving 2 years of adjuvant abemaciclib and adding it to endocrine therapy in patients with high-risk hormone receptor-positive breast cancer. And so, high-risk was defined as having 4 or more positive nodes, or if 1 to 3 positive nodes, either being high grade, or having a tumor that was greater than or equal to 5 centimeters. There was also a second cohort, though, for patients who had 1 to 3 positive nodes but didn't meet the grade or size criteria in cohort 1, and they instead met eligibility because they had a high Ki-67, so they were 1 to 3 positive nodes and high Ki-67, but tumor was under 5 centimeters, and it was not high grade. And so, there were about 5,600 patients that were randomized to get endocrine therapy with or without abemaciclib. And again, the abemaciclib was given for 2 years.
And what we saw was that the 2 years of abemaciclib has led to a significant reduction in invasive disease-free survival events. And in fact, there is now 5 years of follow up from this trial, and at this point, what we've seen is almost a third reduction in events looking specifically at IDFS events. So, the hazard ratio was 0.68, and the absolute difference between the arms was 7.6%. So, clearly a very significant reduction in rates of recurrence from using 2 years of adjuvant abemaciclib.
And so, at this point, the recommendation from multiple different guidelines is to consider abemaciclib in this high-risk population as defined by monarchE. So again, those patients who have 4 more positive nodes, or had 1 to 3 positive nodes and a tumor over 5 centimeters or was high-grade.
So, if we circle back to our patient, again, remember this is a patient who has node-positive ER-positive lobular breast cancer, and she had elected not to get chemotherapy as a pre-menopausal woman with this Oncotype score of 11. And so, now you come back to thinking about whether or not you should add a CDK4/6 inhibitor. Remember, the patient had a tumor that at the time of surgery was actually 4.1 centimeters, was grade 2, and had 2 positive lymph nodes. So, technically not over 5 centimeters. Technically, not high grade. And this is someone with 1 to 3 positive nodes. So, this patient does fall just outside the monarchE eligibility.
And so, I think it becomes a discussion about whether or not you think this patient should get abemaciclib. In my mind, this patient does have risk of recurrence because they have 2 positive nodes, they have a tumor that's almost 5 centimeters and its intermediate grade. And so, I would recommend abemaciclib in this particular patient, even though, again, they technically fell outside that monarchE eligibility. We saw that there was about a third risk reduction from the use of abemaciclib in this setting. This is also a patient who chose to forego adjuvant chemotherapy in a premenopausal population, and so I would be trying to maximize my endocrine therapy.
And so, then you think about, OK, well, if we are trying to maximize our adjuvant systemic treatment, how do we choose which CDK4/6 inhibitor to give? We just reviewed the data from monarchE and showed the benefits of abemaciclib in this setting, but there's also data now from NATALEE, which is a trial that looked at 3 years of adjuvant ribociclib. This trial was designed a little differently than monarchE because it took the standard dose that's used of ribociclib in the metastatic setting and reduced it. So, normally we give 600 milligrams of ribociclib for metastatic disease, this study used 400 milligrams in the adjuvant setting, and it also gave the ribociclib for 3 years. And remember, in monarchE the abemaciclib was for 2 years, so this is a longer duration of therapy. And the eligibility for the trial was broader than the eligibility in monarchE. So, you were eligible if you had any nodal involvement. So, 1 or more positive nodes automatically made you eligible, you didn't need another high-risk feature if you were node positive. But if you were node negative, you did need to have an additional high-risk feature. So, you needed to be at least 2 cm and have either a high Ki-67, or have a high genomic assay score, or be high grade. So, you needed to meet some other eligibility criteria in order to go on to the study if you were node negative.
So again, this is a broader number of patients than are in our monarchE because it's widening the eligibility to include, potentially, T2 and 0 patients and then, any nodal and positive patient.
And we do have data now with 33 months of follow up that has suggested benefit from ribociclib with a hazard ratio of 0.75. So, about a 25% reduction in invasive disease-free survival events, which translated into a 3.1% absolute difference between the 2 arms. The challenges is the data is still early, because about 20% of patients are actually still getting their ribociclib on trial. So, we don't have data for after, when patients have completed their ribociclib, to see that that benefit has continued thereafter.
So, in this particular case, given the longer follow-up time that we have from monarchE with greater maturity of data, it was elected for this patient to go on to abemaciclib. So, she went on to start her abemaciclib, but about 8 days later started experiencing diarrhea and she was having about four bowel movements a day. So, now what are you going to do?
So, unfortunately, diarrhea is a pretty common side effect with abemaciclib. So, we see that about 80% of patients will have some level of diarrhea with abemaciclib, but it is mostly low-grade diarrhea. This is different than ribociclib, which doesn't carry with it the diarrhea, but instead, does have higher rates of hematologic toxicity with more neutropenia and more elevation of liver enzymes. So, the drugs do have different side-effect profiles.
If we dive in deeper in looking at the monarchE toxicity that was seen, again we mentioned that a little over 80% of people have some level of diarrhea, but only about 9% is high-grade diarrhea. The other rare side effects with abemaciclib to keep in mind are that you can see thromboembolic events. So, you can see about 2.5% of patients can develop a thromboembolic event while getting the abemaciclib endocrine therapy, and about 3% of patients can experience interstitial lung disease. And while these are very uncommon side effects, it's important to be aware of them. It's also important to be aware that the rate of thromboembolic events is higher if someone's taking abemaciclib concurrently with tamoxifen, where that rate is about 4%.
So, generally speaking, when someone is having diarrhea like this I would have held therapy. And the question then that comes up is, let's say, you held the therapy, the patient comes back and is doing much better, are you going to dose-modify them? And does dose modification actually carry with it some detriment to efficacy of the abemaciclib? I will say this is probably one of the most common questions my patients ask me is, I'm a little nervous if you reduce the dose, because they're worried that they're going to end up with a higher probability of recurrence and not gain as much benefit from the drug. But in fact, there has been data that has looked at the monarchE data and looked at those patients who had to have dose modifications and looked at efficacy and found no difference in efficacy, whether or not someone had lower dose exposure compared to those patients who maybe had higher dose exposure. And so, to me, this data was very reassuring because it told me that I should feel comfortable dose-modifying because it doesn't seem to impact outcomes here. So, normally with abemaciclib, you treat at 150 milligrams twice daily given on a continuous dosing schedule, but you can dose-modify down to 100 milligrams, or even down to 50 milligrams.
So, in this patient who has diarrhea, again, always making sure when you educate someone prior to starting the abemaciclib that you make sure that they have loperamide on hand, that they are staying hydrated, that they're watching their diet because, certainly, some foods are associated with higher rates of diarrhea. So, important to educate people upfront about this and important to monitor patients.
I do see patients back who are on abemaciclib every 2 weeks for the first 2 months, making sure that I'm checking their blood counts, as well as their liver enzymes. So, very important, again, to have this monitoring in place.
So, for this patient with the diarrhea, it is recommended to hold the abemaciclib, to use the antidiarrheal therapy like loperamide, as needed. And once the diarrhea is resolved, I would usually reinitiate the abemaciclib with dose-modification, usually going from that 150 dose down to 100 mg twice daily.
So, I think the other thing to keep in mind when caring for all our patients is trying to make sure that we address issues surrounding ethnic and racial disparities amongst our minority patients. We do know that being a black patient or of African ancestry is associated with higher rates of distant recurrence and has also been associated with delays in access to care. This is obviously very problematic, and we also know that a lot of our patients who do come from different racial and ethnic backgrounds can have lower rates of screening so sometimes cancers are found at a later stage. And this really, I think is something we need to do better with. And so, it is critical here that we are very good at engaging with our patients, that communication is good, and that education, in my mind, is key. So, for all of our patients we need to make sure that we're really explaining patients what their risk is upfront and making sure that we also explain why they’re taking the medications that they're taking and give them potential strategies, also, to increase adherence. I think this really goes a long way with patients to help them understand what they're doing for treatment, and help them adhere to therapy along the way, and help them build more trust in our medical system.
So, in this particular case, again, a node positive patient who had hormone receptor-positive disease who had a low Oncotype score went on to get ovarian suppression, an AI, and abemaciclib. So, I think a very reasonable treatment approach to try to mitigate that risk of recurrence, but really important to be aware of potential toxicities and educate your patient upfront about them.
When thinking about shared decision-making, it's really important to think about ways that we could do better in educating our patients. And there actually is a downloadable resource that is available to you to serve as a point-of-care reference and patient educational tool that I think will help facilitate providing equitable patient education, and really having a shared decision-making dialogue, so please do take advantage of it.
Thank you so much for your attention.
Announcer Close:
You have been listening to CME on ReachMD. This activity is provided by AXIS Medical Education and is supported by an educational grant from Novartis Pharmaceuticals Corporation.
To receive your free CME credit, or to download this activity, go to reachmd.com/CME. Thank you for listening
 In support of improving patient care, AXIS Medical Education is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
In support of improving patient care, AXIS Medical Education is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. This activity was planned by and for the healthcare team, and learners will receive 0.25 Interprofessional Continuing Education (IPCE) credit for learning and change.
This activity was planned by and for the healthcare team, and learners will receive 0.25 Interprofessional Continuing Education (IPCE) credit for learning and change. AXIS Medical Education has been authorized by the American Academy of PAs (AAPA) to award AAPA Category 1 CME credit for activities planned in accordance with AAPA CME Criteria. This activity is designated for 0.25 AAPA Category 1 CME credits. Approval is valid until 4/29/2025. PAs should only claim credit commensurate with the extent of their participation.
AXIS Medical Education has been authorized by the American Academy of PAs (AAPA) to award AAPA Category 1 CME credit for activities planned in accordance with AAPA CME Criteria. This activity is designated for 0.25 AAPA Category 1 CME credits. Approval is valid until 4/29/2025. PAs should only claim credit commensurate with the extent of their participation. 



Facebook Comments