Alzheimer’s disease (AD) is a progressive neurodegenerative condition that is rapidly increasing in prevalence as the population ages.1 Historically, therapies for AD have been limited to symptom management and a handful of FDA-approved agents designed to marginally reduce cognitive and/or behavioral effects. However, AD recently saw its first approvals for disease-modifying therapy (DMT) with aducanumab in 2021 followed by lecanemab in 2023.2,3 Another late stage investigational agent, donanemab, may not be far behind given the recent announcement of its positive Phase 3 (TRAILBLAZER-ALZ 2) trial.4
These DMTs, all targeting amyloid-β (Aβ), significantly impact the way AD is viewed and managed and introduce several unique considerations into clinical practice. In particular, amyloid-related imaging abnormalities (ARIA), the most common adverse effects seen in DMT trials, are unique to this agent class, raising new questions across multiple medical specialties.
The Modern Understanding of Neuropathology in AD: The Role of Biomarkers
AD pathology is complex and progresses through a spectrum of multiple stages. Though several models of Alzheimer’s staging exist, modern tools to examine AD neurobiology have revealed that its pathology can begin developing decades before symptoms occur, starting the spectrum with an extended preclinical phase.5 The first recognizable, symptomatic stage of the disease is then mild cognitive impairment (MCI) followed, for many, by stages of true Alzheimer’s dementia from mild through severe.
A key discernable pathology thought to drive AD is the accumulation of Aβ protein species and plaques in the brain. According to the amyloid hypothesis, the buildup of Aβ is the dominant pathology in the preclinical and early symptomatic stages of AD before triggering a broader neurotoxic cascade involving several pathologies, the most prominent of which is formation of neurofibrillary tangles containing hyperphosphorylated tau protein,6 (Figure 1). As correlations between tau biomarkers and clinical measures of cognition appear to be more significant than amyloid in the symptomatic phases of AD,7 tau is thought to drive much of the neurodegeneration seen in later stages of the disease.8
FIGURE 1: Goal of Aβ-Targeting DMT6
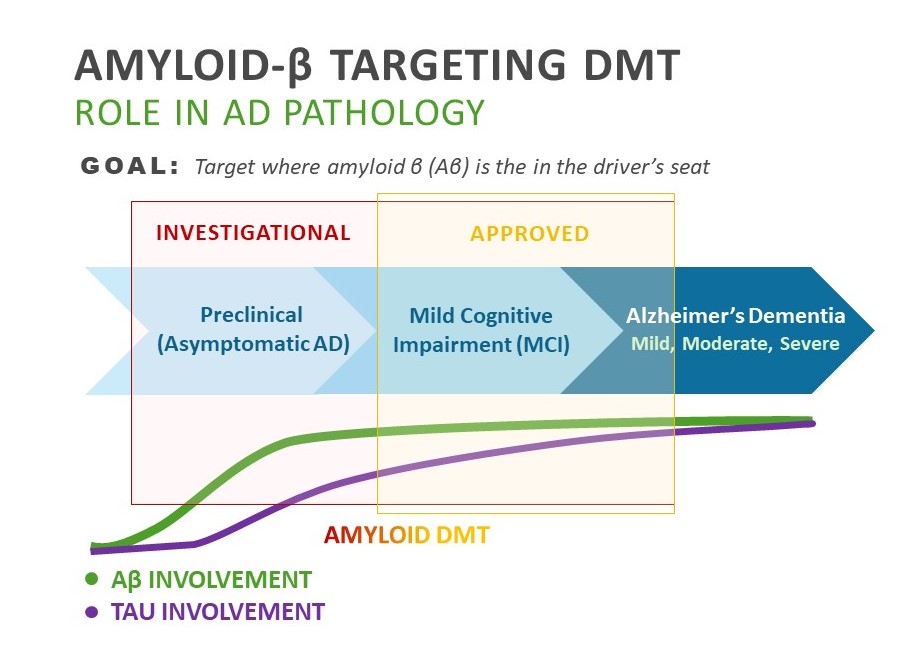
The Rise of Aβ-Targeting DMT
Both aducanumab and lecanemab are approved for the treatment of early symptomatic stages of AD pathology (MCI and early-AD).2,3 Rising phase 3 donanemab also currently targets this range, but both donanemab and lecanemab are being evaluated in ongoing trials targeting Aβ in the preclinical phase as well.8,9 A key goal of all these agents is to remove amyloid while it is still the dominant disease pathology and before it has a chance to induce the greater neurotoxic cascade that manifests as moderate to severe dementia.10
What is the evidence that Aβ DMTs impact AD pathology?
Clinical trials show that significant amyloid clearance was demonstrated by all the approved and late-stage monoclonal antibodies when compared to placebo:
- Aducanumab use (phase 3, EMERGE) showed both a dose and time-dependent reduction in amyloid, with a 71% decrease in amyloid burden on PET for the high dose group.11
- Lecanemab use (phase 2) resulted in a significant decrease in amyloid PET with 81% of participants becoming amyloid negative at 18 months.
- Donanemab use (phase 2) led to 40% of patients becoming amyloid negative at 24 weeks and 68% becoming amyloid negative at 76 weeks.14
What is the evidence that reducing amyloid burden is associated with clinical benefits?
Aducanumab
Though aducanumab was the first approved, its initial phase 3 trial failed its futility analysis, raising questions about the potential clinical benefit of reducing Aβ. However, the trials were subsequently reanalyzed, revealing statistically significant clinical benefit in the EMERGE trial’s high dose group. A particularly important element of this finding was the 40% improvement seen on the ADCS-ADL-MCI (P= 0.006),12 an activities of daily living (ADL) scale designed specifically for MCI. This is thought to more accurately reflect challenges associated with early cognitive decline versus other ADL scales that assess elements more affected at later stages of dementia.
Lecanemab
More recently, topline results from the lecanemab phase 3 trial (CLARITY) revealed a 27% slowing of its primary clinical cognitive outcome, the CDR-SB (P<0.0001),15 over 18 months along with improvement in many others. Such findings led to its approval in January 2023. A notable feature of this trial was that it was conducted in a hierarchical fashion such that its primary outcome had to be met for a participant to be included in the next outcome analysis and so forth. This is thought to make the stated findings more robust.
Donanemab
Adding to the recent influx of data with Aβ-DMT, topline results of donanemab’s phase 3 TRAILBLAZER-ALZ 2 found that donanemab use met all of its primary and secondary endpoints including a slowed clinical decline by 35% compared to placebo (P<0.0001; primary outcome) and 40% less decline in the ability to perform ADLs (P<0.0001; secondary outcome).4 It is also important to note that donanemab trials are the only of the Aβ-DMT group to have included tau staging on top of amyloid positivity in patient selection, potentially providing additional clues as to how to isolate patients who may benefit from this strategy.
All agents
A recent meta-analysis evaluated all outcomes measures of Aβ-DMT trials to date. This analysis of 16 randomized trials found that each 0.1-unit decrease in PET Aβ standardized uptake value ratio (SUVR) was associated with a reduction of a 0.09-, 0.33-, and 0.13-point average change of the CDR-SB, the ADAS-Cog, and the MMSE, respectively. Authors concluded that this was statistically significant evidence that there is likely a causal relationship between a reduction in Aβ plaque and a reduction in cognitive and functional decline.16
New Classes, New Considerations: ARIA
What is the prevalence of ARIA?
ARIA is the most common adverse effect across Aβ DMTs. The prevalence varied between individual agent trials*, but ranged from 13-35% for ARIE-E (edema) and 17-31% for ARIA-H (hemorrhage).14,15,17 Notably, ARIA was also reported in up to 9% of patients in the placebo group,14,15,17 presumably related to the fact that MRI findings associated with ARIA resemeble those of many other causes. For example, microhemorrhages are commonly seen in normal aging or in people with hypertension.
*Note: Trials are not directly comparable due to differences in patient populations and study design
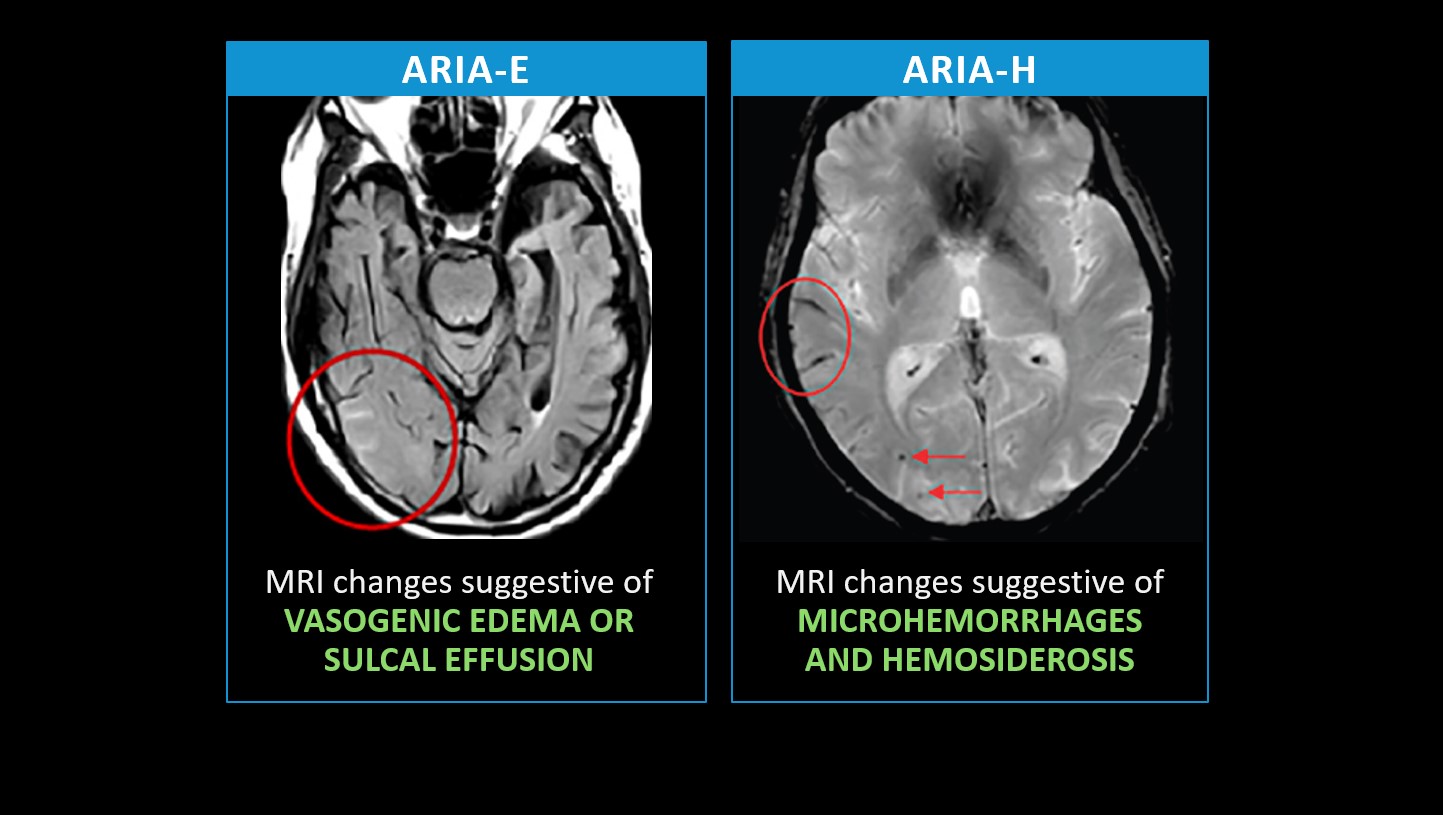
What are the types of ARIA?
As alluded to previously, ARIA can occur in two forms (Figure 2): ARIA with edema or effusion (ARIA-E) or ARIA with hemorrhage (ARIA-H). The example image on the left (ARIA-E) delineates a bright signal within the sulci at the right temporal occipital junction, signifying sulcal effusion.18 Should that infiltrate the brain tissue and parenchyma, it would be considered vasogenic edema. In the example on the right (ARIA-H), gradient echo shows a dark signal in the sulci representing siderosis (or blood products; circle) along with punctate foci of dark signal representing microhemorrhages in the parenchyma (arrows).19
FIGURE 2: Types of ARIA18,19
Who is most at risk for ARIA?
One of the most significant risk factors for ARIA is being an apolipoprotein E ε4 (APOE ε4) genetic carrier, and this risk increases with the number of alleles.19 For example, in aducanumab trials, 64% of homozygotes developed ARIA compared to 35% of heterozygotes and 20% of noncarriers.17 This risk does not preclude use of DMT but may warrant greater precautions. Other risk factors include older age and certain baseline vascular clinical (e.g., cerebrovascular disease) and MRI (e.g., >4 baseline microhemorrhages) characteristics.20
FIGURE 3: How to Gauge ARIA Risk17,19,20
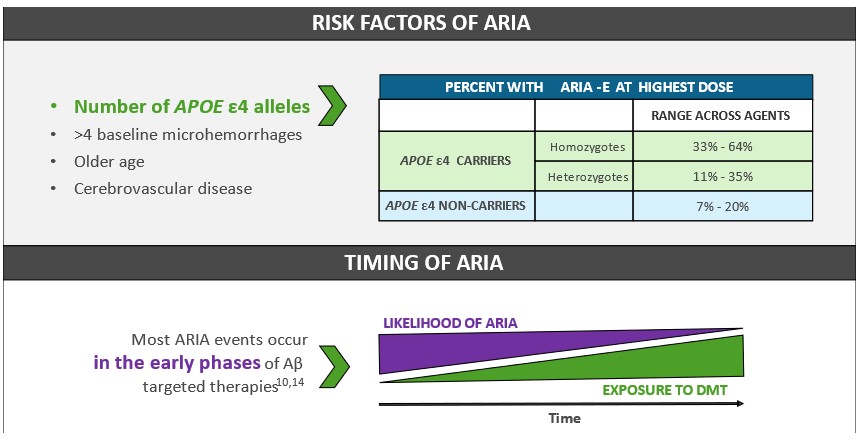
When are patients most at risk for ARIA?
The risk of ARIA is found to be greatest earlier in the treatment phase of Aβ DMT and decreases over time of medication exposure. Enhanced vigilance is recommended during the first ~3-8 months of therapy depending on the specific agent.14,17,21
What does ARIA represent clinically?
Approximately 74% of ARIA is asymptomatic17 and may only be uncovered through the routine MRI monitoring required with Aβ DMT use. But for those that are symptomatic, the most common presentations include headache, dizziness, and altered mental status. Less common symptoms include gait disturbances, nausea, vomiting, visuospatial agnosias, and seizure.17 While asymptomatic ARIA may not require any intervention at all, a select few may result in severe symptoms (e.g., seizure) that require immediate action.21 Watch for the release of modules 2-5 of this series to see how ARIA may present and be managed within your specific specialty setting.















Facebook Comments